

一种氟代丁酸腺苷衍生物的合成
收稿日期: 2013-11-05
修回日期: 2014-01-20
网络出版日期: 2014-03-03
基金资助
国家自然科学基金(No. 81072514)和国家自然青年科学基金(No. 21002067)资助项目.
Rational Design and Synthesis of a Fluorinated Butyric Adenosine Derivative
Received date: 2013-11-05
Revised date: 2014-01-20
Online published: 2014-03-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 81072514) and the National Natural Science Foundation for Young Scientists of China (No. 21002067).
姚后宗 , 尹伟 , 陈晶磊 , 乔春华 . 一种氟代丁酸腺苷衍生物的合成[J]. 有机化学, 2014 , 34(6) : 1222 -1226 . DOI: 10.6023/cjoc201311005
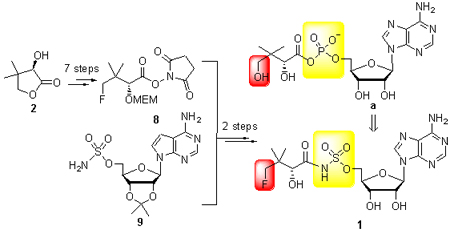
Pantothenate synthetase (PS), the key enzyme in the biosynthesis of the pantothenate acid, is considered to be a new target for anti-tuberculosis drug development. Based on the structure of the enzyme catalyzed reaction intermediate and bioisosterism principle, 5'-O-{[(R)-4-fluoro-2-hydroxy-3,3-dimethylbutanoyl]sulfamoyl}adenosine (1) was designed with the sulfamate isoster to replace the unstable phosphate moiety. The synthesis of this compound was achieved using pantolactone and adenosine as the starting materials through 9 steps of reaction. All the compounds were characterized by 1H NMR, 13C NMR and MS techniques.
Key words: adenosine derivative; pantothenate synthetase; anti-tuberculosis
[1] (a) Mcintosh, E. N.; Purko, M.; Wood, W. A. J. Biol. Chem. 1957, 228, 449.
(b) Yang, Y. H.; Xiao, C. L. Chem. Life 2008, 28, 448 (in Chinese).
(杨延辉, 肖春玲, 生命的化学, 2008, 28, 448.)
[2] Mdluli, K.; Spigelman, M. Curr. Opin. Pharmacol. 2006, 6, 459.
[3] Webb, M. E.; Smith, A. G.; Abell, C. Nat. Prod. Rep. 2004, 21, 695.
[4] Somu, R. V.; Boshoff, H.; Qiao, C.; Bennett, E. M.; Barry III, C. E.; Aldrich, C. C. J. Med. Chem. 2006, 49, 31.
[5] Tuck, K. L.; Saldanha, S. A.; Birch, L. M.; Smith, A. G.; Abell, C. Org. Biomol. Chem. 2006, 4, 3606.
[6] Yan, Z. H.; Lai, K. M.; Tian, W. S.; Yu, Z. X. Acta Chim. Sinica 2012, 70, 1322 (in Chinese).
(严兆华, 赖焜民, 田伟生, 余章昕, 化学学报, 2012, 70, 1322.)
[7] Yang, X. Y.; Lv, T. M.; Zhu, S. J.; Wu, F. H. Acta Chim. Sinica 2011, 69, 815 (in Chinese).
(杨雪艳, 吕铁梅, 朱胜杰, 吴范宏, 化学学报, 2011, 69, 815.)
[8] Kumaki, Y.; Day, C. W.; Smee, D. F.; Morrey, J. D.; Barnard, D. L. Antiviral Res. 2011, 92, 329.
[9] Mandel, A. L.; La Clair, J. J.; Burkart, M. D. Org. Lett. 2004, 6, 4802.
[10] Woo, S. H.; Frechette, S.; Khalil, E. A.; Bouchain, G.; Vaisburg, A.; Bernstein, N.; Moradei, O.; Leit, S.; Allan, M.; Fournel, M.; Trachy-Bourget, M. C.; Li, Z. M.; Besterman, J. M.; Delorme, D. J. Med. Chem. 2002, 45, 2882.
[11] Hon, Y. S.; Wong, Y. C.; Chang, C. P.; Hsieh, C. H. Tetrahedron 2007, 63, 11329.
[12] Neres, J.; Labello, N. P.; Somu, R. V.; Boshoff, H. I.; Wilson, D. J.; Vannada, J.; Chen, L. Q.; Barry III, C. E.; Bennett, E. M.; Aldrich, C. C. J. Med. Chem. 2008, 51, 5357.
[13] Xu, Z. X.; Yin, W.; Chen, J. L.; Qiao, C. H. Chin. J. Org. Chem. 2013, 33, 1578 (in Chinese).
(许芝祥, 尹伟, 陈晶磊, 乔春华, 有机化学, 2013, 33, 1578.)
/
| 〈 |
|
〉 |