

新型茚基膦金配合物的合成及其在炔烃水合反应中的催化作用研究
收稿日期: 2014-01-23
修回日期: 2014-02-22
网络出版日期: 2014-03-12
基金资助
国家自然科学基金(Nos. 21072071,21272088)资助项目.
Synthetic and Structural Studies on Indenyl Phosphine Gold Complexes and Application as Catalyst for Hydration of Alkynes
Received date: 2014-01-23
Revised date: 2014-02-22
Online published: 2014-03-12
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21072071, 21272088).
韩治军 , 毛树兰 , 彭晖 , 皮云宵 , 陈友 , 刘盛华 , 余广鳌 . 新型茚基膦金配合物的合成及其在炔烃水合反应中的催化作用研究[J]. 有机化学, 2014 , 34(5) : 893 -897 . DOI: 10.6023/cjoc201401037
Four novel indenyl phosphine gold complexes were prepared by the reaction of indenyl phosphine with AuCl-(SMe)2. All products 1~4 have been fully characterized by elemental analysis, spectroscopy, and X-ray crystal diffraction techniques for 2 and 4. Complex 2 has been found efficiently to catalyze hydration of alkyne with the advantages of mild reaction conditions, less catalyst, excellent atomic economy and environmental friendly.
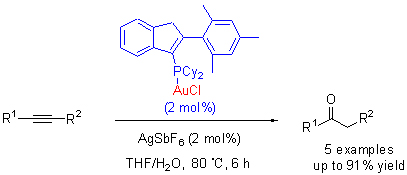
Key words: indenyl; phosphine; gold; alkyne; hydration reaction
[1] Otera, J. Modern Carbonyl Chemistry, Wiley-VCH, Weinheim, Germany, 2000.
[2] Kucherov, M. Chem. Ber. 1881, 14, 1540.
[3] Trost, B. M. Science 1991, 254, 1471.
[4] (a) Hiscox, W.; Jennings, P. W. Organometallics 1990, 9, 1997.
(b) Francisco, L. W.; Moreno, D. A.; Atwood, J. D. Organometallics 2001, 20, 4237.
[5] (a) Tokunaga, M.; Wakatsuki, Y. Angew. Chem., Int. Ed. 1998, 37, 2867.
(b) Suzuki, T.; Tokunaga, M.; Wakatsuki, Y. Org. Lett. 2001, 3, 735.
[6] (a) Janout, V.; Regen, S. L. J. Org. Chem. 1982, 47, 3331.
(b) Tachinami, T.; Nishimura, T.; Ushimaru, R.; Noyori, R.; Naka, H. J. Am. Chem. Soc. 2013, 135, 50.
[7] (a) Fukuda, Y.; Utimoto, K. J. Org. Chem. 1991, 56, 3729.
(b) Marion, N.; Ram?n, R. S.; Nolan, S. P. J. Am. Chem. Soc. 2009, 131, 448.
(c) Wang, D.; Cai, R.; Sharma, S.; Jirak, J.; Thummanapelli, S. K.; Akhmedov, N. G.; Zhang, H.; Liu, X.; Petersen, J. L.; Shi, X. J. Am. Chem. Soc. 2012, 134, 9012.
[8] (a) Nun, P.; Ramón, R. S.; Gaillard, S.; Nolan, S. P. J. Organomet. Chem. 2011, 696, 7.
(b) Leyva, A.; Corma, A. J. Org. Chem. 2009, 74, 2067.
[9] Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239.
[10] Luzung, M. R.; Markham, J. P.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 10858.
[11] Mizushima, E.; Hayashi, T.; Tanaka, M. Org. Lett. 2003, 5, 3349.
[12] Alm?ssy, A.; Nagy, C. E.; Bényei, A. C.; Joó, F. Organometallics 2010, 29, 2484.
[13] (a) Wang, X.; Lai, Y.; Wu, H.; Zhang, J.; Li, Y. Chin. J. Org. Chem. 2009, 29, 432 (in Chinese).
(王翔, 赖媛嫒, 吴宏, 张建明, 李永建, 有机化学, 2009, 29, 432.)
(b) Zhang, Y; Zhu, C. Chin. J. Org. Chem. 2012, 32, 2283. (in Chinese).
(张艳, 朱成建, 有机化学, 2012, 32, 2283.)
[14] Zhang, R.; Xu, Q.; Shi, M. Acta Chim. Sinica 2012, 70, 1593 (in Chinese).
(张睿, 徐琴, 施敏, 化学学报, 2012, 70, 1593.)
[15] Chen, L.; Yu, G. A.; Li, F.; Zhu, X.; Zhang, B.; Guo, R.; Li, X.; Yang, Q.; Jin, S.; Li, C.; Liu, S. H. J. Organomet. Chem. 2010, 695, 1768.
[16] Hao, X.; Yuan, J.; Yu, G.-A.; Qiu, M.-Q.; She, N.-F.; Sun, Y.; Zhao, C.; Mao, S.-L.; Yin, J.; Liu, S.-H. J. Organomet. Chem. 2012, 706, 99.
[17] Mao, S.-L.; Sun, Y.; Yu, G.-A.; Zhao, C.; Han, Z.-J.; Yuan, J.; Zhu, X.; Yang, Q.; Liu, S. -H. Org. Biomol. Chem. 2012, 10, 9410.
[18] Yuan, J.; Sun, Y.; Yu, G.-A.; Zhao, C.; She, N.-F.; Mao, S.-L.; Huang, P.-S.; Han, Z.-J.; Yin, J.; Liu, S. H. Dalton Trans. 2012, 41, 10309.
[19] Hooper, T. N.; Butts, C. P.; Green, M.; Haddow, M. F.; McGrady, J.E.; Russell, C. A. Chem. Eur. J. 2009, 15, 12196.
[20] Goossen, L. J.; Manjolinho, F.; Khan, B. A.; Rodríguez, N. J. Org. Chem. 2009, 74, 2620.
[21] Chen, Z.-W.; Ye, D.-N.; Qian, Y.-P.; Ye, M.; Liu, L.-X. Tetrahedron 2013, 69, 6116.Gatti, N. Tetrahedron Lett. 1990, 31, 3933.
/
| 〈 |
|
〉 |