

新型维甲酸类化合物的合成及生物活性研究
收稿日期: 2014-02-11
修回日期: 2014-03-31
网络出版日期: 2014-04-01
基金资助
上海市教委科研创新项目(No.13ZZ047)、教育部留学归国人员科研启动基金(No.教外司留[2011]1139号)和中央高校基本科研业务费专项资金资助项目.
Synthesis and Biological Evaluation of New Retinoid Derivatives
Received date: 2014-02-11
Revised date: 2014-03-31
Online published: 2014-04-01
Supported by
Project supported by the Innovation Program of Shanghai Municipal Education Commission (No. 13ZZ047), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (No. 2011-1139) and the State Education Ministry and the Fundamental Research Funds for the Central Universities.
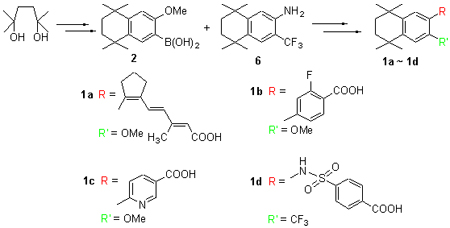
以苯甲醚为起始原料,经Friedel-Crafts反应、溴化和亲核取代转化为硼酸中间体2. 以羟基丙酮12为起始原料,经过Wittig反应、溴化、Arbuzov反应、Horner-Wadsworth-Emmons (HWE)反应后得到共轭烯烃溴代物3; 化合物2分别与化合物3、4-溴-2-氟苯甲酸甲酯(4)和6-溴烟酸甲酯(5)经Suzuki偶联反应和水解反应,得到三个未见报道的维甲酸类似物1a~1c. 同时,以溴苯为起始原料经Friedel-Crafts反应、硝化反应、三氟甲基化反应、硝基还原和亲核取代合成了含有三氟甲基的维甲酸类似物1d. 化合物1a~1d的结构经过1H NMR,13C NMR,IR及HRMS的表征,并对四个化合物进行了HL-60细胞株分化活性能力的测试. 结果表明化合物1a~1d都具有潜在的药物活性,其中含氟维甲酸类似物1b和1d拥有较商品化药物全反式维甲酸(ATRA)更低的IC50值.
徐之涵 , 潘燊 , 黄焰根 . 新型维甲酸类化合物的合成及生物活性研究[J]. 有机化学, 2014 , 34(7) : 1391 -1398 . DOI: 10.6023/cjoc201401047
The key intermediate boronic acid 2 was synthesized via sequential reactions of Friedel-Crafts alkylation, bromination and nucleophilic reaction by using anisole as starting material. Hydroxyacetone was converted to bromide compound 3 by the Wittig reaction, bromination, Arbuzov reaction and Horner-Wadsworth-Emmons (HWE) reaction. Three novel retinoid derivatives 1a~1c can be obtained via the Suzuki coupling reaction of the boronic acid 2 with bromide compound 3, methyl 4-bromo-2-fluorobenzoate (4) or methyl 6-bromonicotinate (5) and followed by hydrolysis of the corresponding esters. In addition, trifluoromethyl substituted retinoid derivative 1d was synthesized via sequential reactions of Friedel-Crafts alkylation, nitration, trifluoromethylation, reduction and nucleophilic reaction with bromobenzene as strarting material. The structures of four new retinoid derivatives 1a~1d were confirmed by 1H NMR, 13C NMR, IR and HRMS analyses. Furthermore, the inducing differentiation abilities of molecules 1a~1d for HL-60 cells were tested, and the results revealed that these four retinoid derivatives are biological active. The IC50 values of fluorinated derivatives 1b and 1d are lower than that of commercial medicine all trans retinoid acid (ATRA).

Key words: retinoid derivative; synthesis; organofluorine compounds; antineoplastic
[1] Barnard, J. H.; Collings, J. C.; Whiting, A.; Przyborski, S. A.; Marder, T. B. Chem. Eur. J. 2009, 15, 11430.
[2] Vaezi, M. F.; Alam, M.; Muccio, D. D.; Rogers, T. S.; Simpson-Herren, L.; Wille, J. J.; Hill, D. L.; Doran, T. I.; Brouillette, W. J.; Muccio, D. D. J. Med. Chem. 1994, 37, 4499.
[3] Alam, M.; Zhestkov, V.; Sani, B. P.; Venepally, P.; Levin, A. A.; Kazmer, S.; Li, E.; Norris, A. W.; Zhang, X. J. Med. Chem. 1995, 38, 2302.
[4] Qing, F. L.; Fan, J. F. Bioorg. Med. Chem. Lett. 1997, 7, 2117.
[5] Qing, F. L.; Fan, J. F. J. Fluorine Chem. 1999, 96, 159.
[6] Qing, F. L.; Yue, X. J. J. Chem. Soc., Perkin Trans. 1 1997, 3053.
[7] Boehm, M. F.; Zhang, L.; Badea, B. A.; White, S. K.; Mais, D. E.; Berger, E.; Suto, C. M.; Goldman, M. E.; Heyman, R. A. J. Med. Chem. 1994, 37, 2930.
[8] Faul, M. M.; Ratz, A. M.; Sullivan, K. A.; Trankle, W. G.; Winneroski, L. L. J. Org. Chem. 2001, 66, 5772.
[9] Farmer, L. J.; Jeong, S.; Kallel, E. A.; Koch, S. S. C.; Croston, G. E.; Flatten, K. S.; Heyman, R. A.; Nadzan A. M. Bioorg. Med. Chem. Lett. 1997, 7, 2393.
[10] Wagner, C. E.; Jurutka, P. W.; Marshall, P. A.; Groy, T. L.; Vaart, A.; Ziller, J. W.; Furmick, J. K.; Graeber, M. E.; Matro, E.; Miguel, B. V.; Tran, I. T.; Kwon, J.; Tedeschi, J. N.; Moosavi, S.; Danishyar, A.; Philp, J. S.; Khamees, R. O.; Jackson, J. N.; Grupe, D. K.; Badshah, S. L.; Hart, J. W. J. Med. Chem. 2009, 52, 5950.
[11] Liu, X. Y.; Li, Z. Z.; Wu, Y. D.; Zhang, Z. Y.; Liang, S. Z.; Jia, X. L.; Xiang, J. N. Acta Chim. Sinica 2008, 66, 1086 (in Chinese).
(刘西洋, 李治章, 吴运东, 张尊英, 梁盛宗, 贾晓雷, 向建南, 化学学报, 2008, 66, 1086.)
[12] Li, W. J.; Nelson, D. P.; Jensen, M. S.; Hoerrner, R. S.; Cai, D. W.; Larsen, R. D.; Reider, P. J. J. Org. Chem. 2002, 67, 5394.
[13] Magoulas, G. E.; Bariamis, S. E.; Athanassopoulos, C. M.; Haskopoulos, A.; Dedes, P. G.; Krokidis, M. G.; Karamanos, N. K.; Kletsas, D.; Papaioannou, D.; Maroulis, G. Eur. J. Med. Chem. 2011, 46, 721.
[14] Miyashita, K.; Sakai, T.; Imanishi, T. Org. Lett. 2003, 5, 2683.
[15] Qing, F.-L.; Qiu, X. Organofluorine Chemistry, Science Press, Beijing, 2007 (in Chinese).
(卿凤翎, 邱小龙, 有机氟化学, 科学出版社, 北京, 2007.)
[16] Chen, Q. Y.; Wu, S. W. J. Chem. Soc., Chem. Commun. 1989, 705.
[17] Zheng, X. X.; Oda, H.; Takamatsu, K.; Sugimoto, Y.; Tai, A.; Akaho, E.; Ali, H. I.; Oshiki, T.; Kakuta, H.; Sasaki, K. Biol. Med. Chem. 2007, 15, 1014.
[18] Hao, B. C.; Zhang, Y. H.; Lai, Y. S.; Yuan, S. T.; Zhang, L. Y. Chin. J. Org. Chem. 2007, 27, 629 (in Chinese).
(郝北辰, 张奕华, 赖宜生, 袁胜涛, 张陆勇, 有机化学, 2007, 27, 629.)
[19] Tsang, K. Y.; Sinha, S.; Liu, X.; Bhat, S.; Chandraratna, R. A. US 20050148590, 2005[Chem. Abstr. 2005, 143, 97206].
[20] Nahoum, V.; Pérez, E.; Germain, P.; Rodr??uez-Barrios, F.; Manzo, F.; Kammerer, S.; Lemaire, G.; Hirsch, O.; Royer, C. A.; Gronemeyer, H.; Lera, A. R.; Bourguet, W. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 17323.
/
| 〈 |
|
〉 |