

通过点击化学方法合成鹅去氧胆酸类分子钳及其识别性能研究
收稿日期: 2014-01-25
修回日期: 2014-03-10
网络出版日期: 2014-04-15
基金资助
四川省科技支撑计划基金(No. 2012SZ0160)和国家外专局基金(No. 2013-32)资助项目.
Synthesis of Molecular Tweezers Derived from Chenodeoxycholic Acid through Click Reaction and Their Recogniton Properties
Received date: 2014-01-25
Revised date: 2014-03-10
Online published: 2014-04-15
Supported by
Project supported by the Science and Technology Department of Sichuan Province (No. 2012SZ0160) and the National Foreign Expert Bureau (No. 2013-32).
赵志刚 , 王晓红 , 石治川 , 程玉宇 . 通过点击化学方法合成鹅去氧胆酸类分子钳及其识别性能研究[J]. 有机化学, 2014 , 34(6) : 1110 -1117 . DOI: 10.6023/cjoc201401042
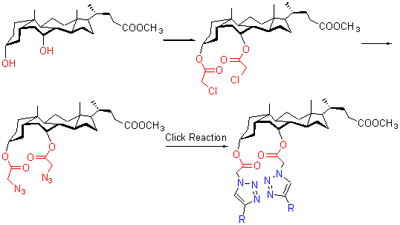
Twelve novel chenodeoxycholic acid-based molecular tweezer metal receptors containing 1,2,3-triazole moieties have been designed and synthesized using click chemistry method. The target compounds were characterized by 1H NMR, IR, MS spectra and elemental analysis. Their binding properties were examined by UV-Vis spectra titration. The results indicate that these molecular tweezers showed high selectivity and affinity for Hg2+ ion.
[1] Clarkson. T. W. Am. J. Clin. Nutr. 1995, 61, 682.
[2] Tchounwou, P. B.; Ayensu, W. K.; Ninashvili, N.; Sutton, D.; Environ. Toxicol. 2003, 18, 149.
[3] An, L.; Cai, Y. H.; Yan, C. G. Chin. J. Appl. Chem. 2005, 22, 980 (in Chinese).
(安琳, 蔡亚华, 颜朝国, 应用化学, 2005, 22, 980.)
[4] Silva, A. P. de; Gunaratne, H. Q. N.; Gunnlaugsson, T.; Huxley, A. J. M.; McCoy, C. P.; Rademacher, J. T.; Rice, T. E. Chem. Rev. 1997, 97, 1515.
[5] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
[6] Demko, Z. P.; Sharpless, K. B. Angew. Chem., Int. Ed., 2002, 41, 2110.
[7] Lewis, W. G.; Green, L. G.; Grynszpan, F.; Radic, Z.; Carlier, P. R.; Taylor, P.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 1053.
[8] Schweinfurth, D.; Hardcastle, K. L.; Bunz, U. H. F. Chem. Commun. 2008, 1053.
[9] Barreto, A. de F. S.; Vercillo, O. E.; Birkett, M. A.; Caulfield, J. C.; Wessjohann, L. A.; Andrade, C. K. Z. Org. Biomol. Chem. 2011, 9, 5024.
[10] Cecioni, S.; Faure, S.; Darbost, U.; Bonnamour, I.; Parrot-Lopez, H.; Roy, O.; Taillefumier, C.; Wimmerova, M.; Praly, J.-P.; Imberty, A.; Vidal, S. Chem. Eur. J. 2011, 17, 2146.
[11] Maeda, C.; Yamaguchi, S.; Ikeda, C.; Shinokubo, H.; Osaka, A. Org. Lett. 2008, 10, 549.
[12] Wang, J.; He, X.; Gao, L.; Sheng, L.; Shi, X,; Li, J.; Chen, G. Chin. J. Chem. 2011, 29, 1227.
[13] Fazio, M. A.; Lee, O. P.; Schuster, D. I. Org. Lett. 2008, 10, 4979.
[14] Lowe, A. J.; Long, B. M.; Pfeffer, F. M. Chem. Commun. 2013, 49, 3376.
[15] Oh, H.; Han, S. K.; Kim. B. H. Heteroat. Chem. 2012, 23, 187
[16] Hu, J.; Lu, J. R.; Ju, Y. Chem. Asian J. 2011, 6, 2636.
[17] Verzele, D.; Madder, A. Eur. J. Org. Chem. 2013, 673.
[18] Jadhav, J. R.; Bae, C. H.; Kim. H. S. Tetrahedron Lett. 2011, 52, 1623.
[19] Li, W. N.; Xu, Q.; Li, Y.; Zhu, W.; Cui, J. C.; Ju, Y.; Li, G. T. Tetrahedron Lett. 2013, 54, 3868.
[20] Li, D. Z.; Yang, Y. X.; Yang, C.; Hu, B. W.; Huo, B. L.; Xue, L. W.; Wang, A. Z. Tetrahedron 2014, 70, 1223.
[21] Hu, J.; Zhang, M.; Yu, L. B.; Ju. Y. Bioorg. Med. Chem. Lett. 2010, 20, 4342
[22] Kumar, A.; Pandey, P. S. Tetrahedron Lett. 2009, 50, 5842.
[23] Benesi, H. A.; Hildebrand, J. H. J. Am. Chem. Soc. 1949, 71, 2703.
/
| 〈 |
|
〉 |