

铜催化一锅法合成吡咯[1,2-a]喹喔啉衍生物
收稿日期: 2014-03-10
修回日期: 2014-04-17
网络出版日期: 2014-05-06
基金资助
国家自然科学基金(No. 21072095)和江苏省研究生创新工程(No. CXLX13_433)资助项目.
One-Pot Synthesis of Pyrrolo[1,2-a]quinoxaline Derivatives through Copper-Catalyzed Cascade Reactions
Received date: 2014-03-10
Revised date: 2014-04-17
Online published: 2014-05-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 21072095), the Graduate Student Innovation Project in Jiangsu Province (No. CXLX13_433).
建立了一种简单实用、经济高效的以邻碘苯胺、吡咯甲醛为起始原料,廉价的CuI为催化剂,价廉、易获得的2,2-联吡啶为配体,110 ℃条件下一锅法合成吡咯[1,2-a]喹喔啉类衍生物的催化体系,得到中等到优良产率. 本方法具有催化剂廉价易得、操作简便、合成效率高的优点.
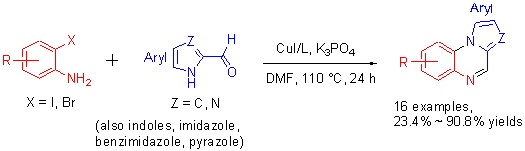
关键词: 一锅法合成; 吡咯[1,2-a]喹喔啉; 铜催化
蒋增强 , 张杰 , 童耀 , 史雪松 , 苗大状 , 韩世清 . 铜催化一锅法合成吡咯[1,2-a]喹喔啉衍生物[J]. 有机化学, 2014 , 34(9) : 1845 -1850 . DOI: 10.6023/cjoc201403022
A simple, efficient, inexpensive one-pot method for the pyrrolo[1,2-a]quinoxaline derivatives synthesized from 2-formylazoles and o-aminoiodoarenes was developed by using cheap CuI as catalyst, inexpensive and readily available 2,2'-bipyridine as ligand at 110 ℃. The reaction provides an efficient protocol to a series of pyrrolo[1,2-a]quinoxaline derivatives in good to high yields, and benefits of readily available catalyst and synthesis of high efficiency.
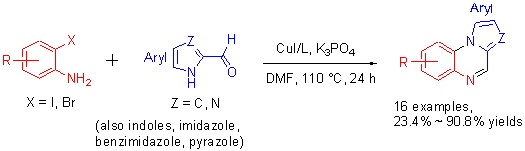
Key words: one-pot synthesis; pyrrolo[1,2-a]quinoxalines; copper-catalyst
[1] (a) Guillon, J.; Dumoulin, H.; Dallemagne, P.; Rault, S.Pharm.Pharmacol.Commun.1998, 4, 33.(b) Guillon, J.; Boulouard, M.; Rault, S.J.Pharm.Pharmacol.2000, 52, 1369.(c) Guillon, J.; Dallemagne, P.; Rault, S.Eur.J.Med.Chem.1998, 33, 293.(d) Gemma, S.; Colombo, L.; Tripaldi, P.Org.Biomol.Chem.2011, 9, 5137.
[2] (a) Alleca, S.; Corona, P.; La Colla, P.Farmaco 2003, 58, 639.(b) Guillon, J.; Forfar, I.; Dufaure, B.Bioorg.Med.Chem.2007, 15, 194.(c) Guillon, J.; Grellier, P.; Péhourcq, F.J.Med.Chem.2004, 47, 1997.(d) Guillon, J.; Mouray, E.; Le-Naour, A.Eur.J.Med.Chem.2011, 46, 2310.(e) Guillon, J.; Moreau, S.; Jarry, C.Bioorg.Med.Chem.2008, 16, 9133.
[3] (a) Morelli, E.; Gemma, S.; Budriesi, R.; Borrelli, G.J.Med.Chem.2009, 52, 3548.(b) Prunier, H.; Rault, S.; Guardiola-Lemaitre, B.J.Med.Chem.1997, 40, 1808.(c) Butini, S.; Budriesi, R.; Hamon, M.; Ioan, P.J.Med.Chem.2009, 52, 6946.
[4] Cheeseman, G.; Tuck, B.Chem.Ind.1965, 1382.
[5] Biswas, S.; Batra, S.Eur.J.Org.Chem.2013, 4895.
[6] Yuan, Q.; Ma, D.J.Org.Chem.2008, 73, 5159.
[7] Reeves, J.T.; Fandrick, D.R.; Senanayake, C.H.J.Org.Chem.2010, 75, 992.
[8] Pereira, M.D.F.; Thiéry, V.R.Org.Lett.2012, 14, 4754.
[9] Huang, A.P.; Chen, Y.M.; Ma, C.Org.Lett.2013, 21, 5480.
[10] (a) Yin, H.Q.; Jin, M.; Chen, W.; Chen, C.; Zheng, L.K.; Wei, P.; Han, S.Q.Tetrahedron Lett.2012, 53, 1265.(b) Zhang, J.X.; Yin, H.Q.; Han, S.Q.Chin.J.Org.Chem.2012, 32, 1429 (in Chinese).(张敬先, 殷慧清, 韩世清, 有机化学, 2012, 32, 1429.) (c) He, G.Z.; Huang, Y.; Tong, Y.; Han, S.Q.Tetrahedron Lett.2013, 54, 5318.(d) Huang, Y.; Chen, C.; Zhao, D.; Han, S.Q.Chin.J.Chem.2013, 31, 1007.(e) Jin, M.; Zhao, D.; He, G.Z.; Han, S.Q.Chin.J.Catal.2013, 34, 1651.
[11] Ste'phanie, P.; Parra, S.Eur.J.Med.Chem.2001, 36, 255.
[12] Bergman, J.; Vallberg, H.Acta Chem.Scand.1997, 51, 742.
[13] Albini, A.; Bettinetti, G.; Minoli, G.J.Am.Chem.Soc.1997, 119, 7308.
/
| 〈 |
|
〉 |