

多手性中心联萘-2-芳甲羟基-2’-醇(Ar-BINMOLs):一类可应用于不对称催化反应的新型手性配体
收稿日期: 2014-03-25
修回日期: 2014-04-17
网络出版日期: 2014-05-06
基金资助
国家自然科学基金(No.21173064)和浙江省自然科学基金(No.LR14B03001)资助项目.
1,1’-Binaphthalene-2-α-arylmethanol-2’-ols (Ar-BINMOLs) with Axial and sp3-Central Chirality:A Novel Chiral Ligands for Catalytic Asymmetric Transformations
Received date: 2014-03-25
Revised date: 2014-04-17
Online published: 2014-05-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 21173064) and the Zhejiang Provincial Natural Science Foundation of China (No. R14B030003).
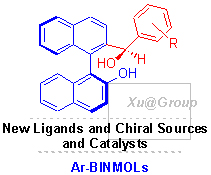
近年来,我们基于1,1’-联萘基-2,2’-二酚(BINOL)为手性源成功地发展了一类多手性中心化合物联萘-2-芳甲羟基-2’-醇(Ar-BINMOLs),它是BINOL的单苄醚化合物经过不对称[1,2]-Wittig重排合成得到的,涉及C-O键的断裂与新C-C的构建反应,由轴手性诱导产生新的碳手性中心,结构独特新颖,同时具有C2轴手性和sp3碳手性的光学纯醇酚类化合物. 目前,Ar-BINMOLs作为新型手性配体应用于不对称催化反应已有不少的报道. 基于BINOL衍生物以及近几年Ar-BINMOLs及其衍生物的研究进展,重点阐述了其作为新型手性配体或手性骨架在不对称催化反应中的应用研究进展.
郑龙生 , 宋涛 , 徐利文 . 多手性中心联萘-2-芳甲羟基-2’-醇(Ar-BINMOLs):一类可应用于不对称催化反应的新型手性配体[J]. 有机化学, 2014 , 34(7) : 1255 -1267 . DOI: 10.6023/cjoc201403053
1-(2-Hydroxynathalen-1-yl)naphthalene-2-ol (BINOL) and its derivatives have been used widely as chiral ligands in asymmetric catalysis. Recently, we have found that the simple axial chiral monoalkylated BINOLs could be converted into synthetically useful and enantiomerically pure 1,1'-binaphthalene-2-α-arylmethanol-2'-ols (Ar-BINMOLs) with axial and sp3-central chirality through the axial-to-central chirality transfer of [1,2]-Wittig rearrangement. At present, the catalytic application of Ar-BINMOLs in asymmetric catalysis has been revealed by our and Yus's groups. In this account, the progress in the field of BINOL and its derivatives will also be reported concisely in the article, and the Ar-BINMOLs and its derivatives as new chiral ligands for application in asymmetric catalysis would be summarized detailedly.

[1] Ager, D. J.; East, M. B. Asymmetric Synthetic Methodology, CRC, Boca Raton, FL, 1996.
[2] Ojima, I. Catalytic Asymmetric Synthesis, John Wiley & Sons, New Jersey, 2004.
[3] Gawley, R. E.; Aubé, J. Principles of Asymmetric Synthesis, 2nd ed, Elsevier, 2012.
[4] Ding, K. L.; Han, Z. B.; Wang, Z. Chem. Asian J. 2009, 4, 32.
[5] Yong, T. P.; Jacobsen, E. N. Science 2003, 299, 1691.
[6] Kagan, H. B. In Asymmetric Synthesis, Ed.: Morrison, J. D., Academic Press, New York, 1985.
[7] Cram, D. J.; Cram, J. M. Acc. Chem. Res. 1978, 11, 8.
[8] Noyori, R.; Tomino, I.; Tanimoto, Y. J. Am. Chem. Soc. 1979, 101, 3129.
[9] Chen, Y.; Yekta, S.; Yudin, A. K. Chem. Rev. 2003, 103, 3155.
[10] Brunel, J. M. Chem. Rev. 2007, 107, 1.
[11] Kamikawa, K.; Watanabe, T.; Uemura, M. J. Org. Chem. 1996, 61, 1375.
[12] Burke, M. D.; Schreiber, S. L. Angew. Chem., Int. Ed. 2004, 43, 468.
[13] Lee, J. Y.; Miller, J. J.; Hamilton, S. S.; Sigman, M. S. Org. Lett. 2005, 7, 1837.
[14] (a) Gao, G.; Gu, F. L.; Jiang, J. X.; Jiang, K. Z.; Sheng, C. Q.; Xu, L. W. Chem. Eur. J. 2011, 17, 2698.
(b) Gao, G.; Bai, X. F.; Yang, H. M.; Jiang, J. X.; Lai, G. Q.; Xu, L. W. Eur. J. Org. Chem. 2011, 5039.
(c) Gao, G.; Bai, X. F.; Li, F.; Zheng, L. S.; Zheng, Z. J.; Lai, G. Q.; Jiang, K. Z.; Li, F. W.; Xu, L. W. Tetrahedron Lett. 2012, 53, 2164.
(d) Zheng, L. S.; Jiang, K. Z.; Deng, Y.; Bai, X. F.; Gao, G.; Gu, F. L.; Xu, L. W. Eur. J. Org. Chem. 2013, 4, 748.
[15] (a) Wu, T. R.; Shen, L. X.; Chong, J. M. Org. Lett. 2004, 6, 2701;
(b) Blay, G.; Fernández, I.; Pedro, R. J.; Vila, C. Org. Lett. 2007, 9, 2601.
(c) Blay, G.; Fernández, M.; Muñoz, C.; Pedro, J. R.; Vila, C. Eur. J. Org. Chem. 2013, 1902.
(d) Huang, G. C.; Yin, Z. S.; Zhang, X. G. Chem. Eur. J. 2013, 19, 11992.
[16] (a) Lingenfelter, D. S.; Helgeson, R. C.; Cram, D. J. J. Org. Chem. 1981, 46, 393.
(b) Cox, P. J.; Wang, W.; Snieckus, V. Tetrahedron Lett. 1992, 33, 2253.
(c) Simonsen, K. B.; Gothelf, K. V.; Jørgensen, K. A. J. Org. Chem. 1998, 63, 7536.
(d) Zhang, Z. G.; Dong, Z. B.; Li, J. S. Chirality 2010, 22, 820.
(e) Lin, L.; Zhang, J. L.; Ma, X. J.; Fu, X.; Wang, R. Org. Lett. 2011, 13, 6410.
[17] Milburn, R. R.; Hussain, S. M. S.; Prien, O.; Ahmed, Z.; Snieckus, V. Org. Lett. 2007, 9, 4403.
[18] (a) Wang, Q.; Chen, S. Y.; Yu, X. Q.; Pu, L. Tetrahedron 2007, 63, 4422.
(b) Yue, Y.; Turlington, M.; Yu, X. Q.; Pu, L. J. Org. Chem. 2009, 74, 8681.
[19] Maruoka, K.; Itoh, T.; Araki, Y.; Shirasaka, T.; Yamamoto, H. Bull. Chem. Soc. Jpn.1988, 8, 2975.
[20] Gribkov, D. V.; Hultzsch, K. C.; Hampel, F. J. Am. Chem. Soc. 2006, 128, 3748.
[21] Kodama, H.; Ito, J.; Hori, K.; Ohta, T.; Furukawa, I. J. Organomet. Chem. 2000, 603, 6.
[22] Beckendorf, S.; Mancheño, O. G. Synthesis 2012, 2162.
[23] Casas, J.; Baeza, A.; Sansano, J. M.; Nájera, C.; Saá, J. M. Tetrahedron: Asymmetry 2003, 14, 197.
[24] Qin, Y. C.; Liu, L.; Sabat, M.; Pu, L. Tetrahedron 2006, 62, 9335.
[25] Gou, S. H.; Zhou, X.; Wang, J.; Liu, X. H.; Feng, X. M. Tetrahedron Lett. 2008, 64, 2864.
[26] Nájera, C.; Sansano, J. M.; Saá, J. M. Eur. J. Org. Chem. 2009, 2385.
[27] Li, H.; Da, C. S.; Xiao, Y. H.; Li, X.; Su, Y. N. J. Org. Chem. 2008, 73, 7398.
[28] Guo, Q. S.; Liu, B.; Lu, Y. N.; Jiang, F. Y.; Song, H. B.; Li, J. S. Tetrahedron: Asymmetry 2005, 16, 3667.
[29] (a) Matsui, K.; Takizawa, S.; Sasai, H. J. Am. Chem. Soc. 2005, 127, 3680.
(b) Matsui, K.; Tanaka, K.; Horii, A.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2006, 17, 578.
[30] Qian, C.; Zhu, C.; Huang, T. J. Chem. Soc., Perkin Trans. 1 1998, 2131.
[31] (a) Vogl, E. M.; Matsunaga, S.; Kanai, M.; Iida, T.; Shibasaki, M. Tetrahedron Lett.1998, 39, 7917.
(b) Matsunaga, S.; Ohshima, T.; Shibasaki, M. Adv. Synth. Catal. 2002, 344, 3.
(c) Shibasaki, M.; Matsunaga, S. J. Organomet. Chem. 2006, 691, 2089.
(d) Shibasaki, M.; Matsunaga, S. Chem. Soc. Rev. 2006, 35, 269.
[32] Hashimoto, T.; Omote, M.; Maruoka, K. Org. Biomol. Chem. 2008, 6, 2263.
[33] (a) Sasai, H.; Arai, R.; Satow, Y.; Houk, K. N.; Shibasaki, M. J. Am. Chem. Soc. 1995, 117, 6194.
(b) Sasai, H.; Tokugana, T.; Shizue, W.; Suzuki, T.; Itoh, N.; Shibasaki, M. J. Org. Chem.1995, 60, 7388.
[34] Kadiri, M. Y. E.; Framery, E.; Andrioletti, B. Tetrahedron Lett. 2012, 53, 6335.
[35] Bunzen, J.; Bruhn, T.; Bringmann, G.; Lützen, A. J. Am. Chem. Soc. 2009, 131, 3621.
[36] Tian, Y.; Chan, K. S. Tetrahedron Lett. 2000, 41, 8813.
[37] Mikami, K.; Ueki, M.; Matsumoto, Y.; Terada, M. Chirality 2001, 13, 541.
[38] Enev, V.; Ewers, C. L. J.; Harre, M.; Nikische, K.; Mohr, J. T. J. Org. Chem. 1997, 62, 7092.
[39] Kiyooka, S.; Tsutsui, T.; Kira, T. Tetrahedron Lett. 1996, 37, 8903.
[40] (a) Marshall J. A. In Comprehensive Organic Synthesis, Vol. 3, Eds.: Trost, B. M.; Fleming, I., Pergamon, Oxford, 1991, p. 975.
(b) Tomooka, K.; Yamamoto, H.; Nakai, T. Liebigs Ann. Recl. 1997, 1275.
[41] Wittig, G.; Löhmann, L. Justus Liebigs Ann. Chem. 1942, 550, 260.
[42] (a) Nakai, T.; Mikami, K. Chem. Rev. 1986, 86, 885.
(b) Kita-mura, M.; Hirokawa, Y.; Maezaki, N. Chem. Eur. J. 2009, 15, 9911.
(c) Sasaki, M.; Ikemoto, H.; Kawahata, M.; Yamaguchi, K.; Takeda, K. Chem. Eur. J. 2009, 15, 4663.
(d) Giampietro, N. C.; Wolf, J. P. Angew. Chem., Int. Ed. 2010, 49, 2922.
[43] Selected examples: (a) Tomooka, K.; Yamamoto, H.; Nakai, T. J. Am.Chem. Soc. 1996, 118, 3317;
(b) Barluenga, J.; Faňanás, F. J.; Sanz, R.; Marcos, C.; Trabada, M. Org. Lett. 2002, 4, 1587.
[44] Santos, L. S. Reactive Intermediates, Wiley-VCH, Weinheim, 2010.
[45] Gao, G. M.S. Thesis, Hangzhou Normal University, Hangzhou, 2012 (in Chinese).
(高广, 硕士论文, 杭州师范大学, 杭州, 2012.)
[46] (a) Somanathan, R.; Flores-Lopez, L.Z.; Montalvo-Gonza-lez, R.; Chavez, D.; Parra-Hake, M.; Auirre, G. Mini-Rev. Org. Chem. 2010, 7, 10.
(b) Zhong, J. C.; Hou, S. C.; Qian, Q. H.; Yin, M. M.;Na, R. S.; Zheng, B.; Li, Z. Y.; Liu, S. Z.; Wang, M. Chem. Eur. J. 2009, 15, 3069.
(c) Lombardo, M.; Chia-rucci, M.; Trombini, C. Chem. Eur. J. 2008, 14, 11288.
(d) Pu, L.; Yu, H. B. Chem. Rev. 2001, 101, 757.
[47] (a) Yoshioka, M.; Kawakita, T.; Ohno, M. Tetrahedron Lett. 1989, 30, 1657.
(b) Takahashi, H.; Kawakita, T.; Yoshioka, M.; Kobayashi, S.; Ohno, M. Tetrahedron Lett. 1989, 30, 7095.
[48] (a) Schmidt, B.; Seebach, D. Angew. Chem., Int. Ed. Engl. 1991, 30, 1321.
(b) Zhang, F. Y.; Yip, C. W.; Cao, R.; Chan, A. S. C. Tetrahedron: Asymmetry 1997, 8, 585.
(c) Balsells, J.; Walsh, P. J. J. Am. Chem.Soc. 2000, 122, 3250.
(d) Balsells, J.; Walsh, P. J. J. Am. Chem.Soc. 2000, 122, 1802.
(e) Hatano, M.; Miyamoto, T.; Ishihara, K. Adv. Synth. Catal. 2005, 347, 1561.
(f) Tanaka, T.; Sano, Y.; Hayashi, M. Chem. Asian J. 2008, 3, 1465.
(g) Gou, S. H.; Judeh, Z. M. A. Tetrahedron Lett. 2009, 50, 281.
(h) Zhang, Z. G.; Dong, Z. B.; Li, J. S. Chirality 2010, 22, 820.
(i) Adreu, A. R.; Pereira, M. M.; Bayon, J. C. Tetrahedron 2010, 66, 743.
(j) Von Ronn, R.; Christoffers, J. Tetrahedron Lett. 2011, 67, 334.
[49] (a) Fernández-Mateos, E.; Maciá, B.; Ramón, D. J.; Yus, M. Eur. J. Org. Chem. 2011, 6851.
(b) Fernández-Mateos, E.; Maciá, B.; Yus, M. Eur. J. Org. Chem. 2012, 3732.
(c) Fernández-Mateos, E.; Maciá, B.; Yus, M. Tetrahedron: Asymmetry 2012, 23, 789.
(d) Fernández-Mateos, E.; Maciá, B.; Yus, M. Adv. Synth. Catal. 2013, 355, 1249.
[50] Li, F.; Zheng, Z. J.; Shang, J. Y.; Jiang, K. Z.; Lai, G. Q.; Jiang, J. X.; Xu, L. W. Chem. Asian J. 2012, 7, 2008.
[51] Li, F.; Li, L.; Yang, W.; Zheng, L. S.; Zheng, Z. J.; Jiang, K. Z.; Lu, Y. X.; Xu, L. W. Tetrahedron Lett. 2013, 54, 1584.
[52] Zheng, L. S.; Li, L.; Yang, K. F.; Zheng, Z. J.; Xiao, X. Q.; Xu, L. W. Tetrahedron Lett. 2013, 69, 8777.
[53] Yamamoto, H.; Zhou, F. Synfacts 2014, 10, 56.
[54] Ye, F.; Zheng, Z. J.; Deng, W. H.; Zheng, L. S.; Deng, Y.; Xia, C. G.; Xu, L. W. Chem. Asian J. 2013, 8, 2242.
[55] Song, T.; Zheng, L. S.; Ye, F.; Deng, W. H.; Wei, Y. L.; Jiang, K. Z.; Xu, L. W. Adv. Synth. Catal. 2014, 356, 1708.
/
| 〈 |
|
〉 |