

3-吲哚基-3-甲硫基丙烯酸酯和3-吲哚基-3-氧代丙酸酯的选择性合成
收稿日期: 2014-03-25
修回日期: 2014-04-17
网络出版日期: 2014-05-06
基金资助
国家自然科学基金(No. 20272008)和鞍山市科技基金(Nos. 2011MS44 和 2012KJ02)资助项目.
Selective Synthesis of 3-(3-Indolyl)-3-(methylthio)acrylate and 3-(3-Indolyl)-3-oxopropanoate
Received date: 2014-03-25
Revised date: 2014-04-17
Online published: 2014-05-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 20272008) and the Science Foundation of Anshan City (Nos. 2011MS44, 2012KJ02).
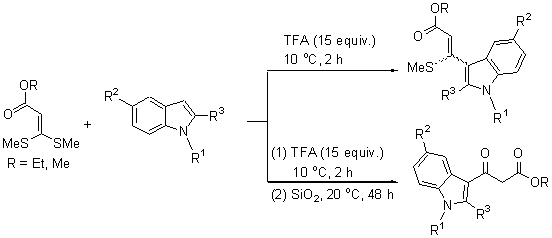
在10 ℃,3,3-二甲硫基丙烯酸酯、吲哚和三氟乙酸(TFA)的物质的量之比为1:1:15时,研究了3,3-二甲硫基丙烯酸酯的吲哚化反应. 通过控制产物在硅胶的停留时间选择性制备了3-吲哚基-3-甲硫基丙烯酸酯(83%~85%)和3-吲哚基-3-氧代丙酸酯(83%~89%). 反应条件温和、操作简单、产率高且选择性好.
关键词: α-羰基二硫缩烯酮; 3-吲哚基-3-烷硫基丙烯酸酯; 3-吲哚基-3-氧代丙酸酯; 吲哚衍生物; 选择性合成
于海丰 , 廖沛球 , 刁全平 , 李铁纯 , 辛广 , 韩立楠 , 侯冬岩 . 3-吲哚基-3-甲硫基丙烯酸酯和3-吲哚基-3-氧代丙酸酯的选择性合成[J]. 有机化学, 2014 , 34(9) : 1851 -1856 . DOI: 10.6023/cjoc201403055
The indolylation reaction of 3,3-bis(methylthio)acrylates was performed at 10 ℃ when the molar ratio of 3,3-bis(methylthio)acrylates, indoles and trifluoroacetic acid (TFA) was 1:1:15. It showed that 3-(3-indolyl)-3-(methyl- thio)acrylate and 3-(3-indolyl)-3-oxopropanoate could be selectively obtained in excellent yield by controlling the detention time of 3-(3-indolyl)-3-(methylthio)acrylate in the silica gel. This transformation is characterized by mild reaction conditions, simple procedure, good yield, and perfect selectivity.

[1] Nicolaou, K.C.; Snyder, S.A.Classics in Total Synthesis II, Wiley-VCH, Weinheim, 2003.
[2] Agarwal, A.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S.Bioorg.Med.Chem.Lett.2005, 15, 3133.
[3] Ma, S.M.; Yu, S.C.Org.Lett.2005, 7, 5063.
[4] Grimster, N.P.; Gauntlett, C.; Godfrey, C.R.A.Angew.Chem., Int.Ed.2005, 44, 3125.
[5] Wang, W.Q.; Ikemoto, T.Tetrahedron Lett.2005, 46, 3875.
[6] Zhang, D.T.; Wang, G.T.; Tan, C.B.; Xu, W.R.; Pei, Y.; Huo, L.Y.Arch.Pharm.Res.2011, 34, 343.
[7] Diana, P.; Carbone, A.; Barraja, P.; Kelter, G.; Fiebig, H.H.; Cirrincione, G.Bioorg.Med.Chem.2010, 18, 4524.
[8] Bergean, J.Acta Chem.Scand.1968, 22, 1063.
[9] Pan, L.; Liu, Q.Synlett 2011, 1073.
[10] Junjappa, H.; Ila, H.; Asokan, C.V.Tetrahedron 1990, 46, 5423.
[11] Dieter, R.K.Tetrahedron 1986, 42, 3029.
[12] Zhang, L.; Liang, F.S.; Cheng, X.J.Org.Chem.2009, 74, 899.
[13] Bi, X.H.; Dong, D.W.; Liu Q.J.Am.Chem.Soc.2005, 127, 4578.
[14] Wang, M.; Sun, S.G.; Liang, D.Q.Eur.J.Org.Chem.2011, 13, 2466.
[15] Liang, D.Q.; Wang, M.; Bekturhun, B.Adv.Synth.Catal.2010, 352, 1593.
[16] Yu, H.F.; Jin, W.W.; Sun, C.L.Angew.Chem., Int.Ed.2010, 49, 5792.
[17] Jin, W.W.; Du, W.M.; Yang, Q.Org.Lett.2011, 13, 4272.
[18] Dong, Y.; Wang, M.; Liu, J.Chem.Commun.2011, 47, 7380.
[19] Yu, H.F.Synth.Commun.2013, 43, 1280.
[20] Yu, H.F.; Liao, P.Q.Chemistry 2013, 76, 435 (in Chinese).(于海丰, 廖沛球, 化学通报, 2013, 76, 435.)
[21] Yu, H.F.Chin.J.Chem.2012, 23, 367.
[22] Yu, H.F.; Liao, P.Q.Chem.J.Chin.Univ.2012, 33, 1969 (in Chinese).(于海丰, 廖沛球, 高等学校化学学报, 2012, 33, 1969.)
[23] Yu, H.F.; Wang, D.L.; Zhao, H.; Hou, D.Y.Chin.J.Org.Chem.2011, 31, 1949 (in Chinese).(于海丰, 王东来, 赵辉, 侯冬岩, 有机化学, 2011, 31, 1949.)
[24] Yu, H.F.; Yu, Z.K.Angew.Chem., Int.Ed.2009, 48, 2929.
[25] Yu, H.F.; Li, T.C.; Liao, P.Q.Synthesis 2012, 44, 3743.
[26] Yu, H.F.; Li, T.C.; Liao, P.Q.; Xin, G.; Hou, D.Y.Chin.J.Org.Chem.2014, 34, 956 (in Chinese).(于海丰, 李铁纯, 廖沛球, 辛广, 侯冬岩, 有机化学, 2014, 34, 956.)
/
| 〈 |
|
〉 |