

酸敏感连接单元的合成及其在两亲性嵌段共聚物中的应用
收稿日期: 2014-03-12
修回日期: 2014-04-28
网络出版日期: 2014-05-07
基金资助
国家自然科学基金(Nos. 81071250, 91227109)和新药创制重大专项(No. 2011ZX09501-001-05)资助项目.
Synthesis and Application of pH-Sensitive Linkers in Amphiphilic Block Copolymer
Received date: 2014-03-12
Revised date: 2014-04-28
Online published: 2014-05-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 81071250, 91227109), ant the Major Projects in National Science and Technology, “Creation of Major New Drugs” (No. 2011ZX09501-001-05).
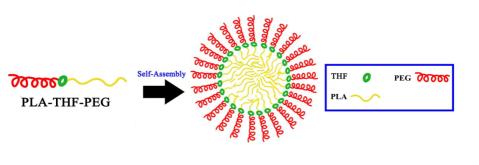
合成了一类基于四氢吡喃/四氢呋喃醚结构的酸敏感连接单元1a和1b,该连接单元的两端均为易反应的活性官能团,其结构经1H NMR、13C NMR、IR和HRMS表征,并合成了一种含四氢吡喃醚结构的小分子嵌段共聚物模型化合物以及含四氢呋喃醚结构的两亲性嵌段共聚物,其中聚乙二醇(PEG)为亲水片段,聚乳酸(PLA)为疏水片段,其结构经1H NMR、IR以及GPC的表征,并测定了该嵌段共聚物自组装胶束的粒径大小、临界胶束浓度(CMC)以及常温下的稳定性. 实验结果表明该类酸敏感嵌段共聚物自组装胶束具有良好的发展前景.
关键词: 四氢吡喃醚/四氢呋喃醚酸敏感连接单元; 两亲性嵌段共聚物; 自组装胶束
朱方霞 , 庄园 , 黎庆 , 杨晴来 , 伍新燕 , 邵志峰 , 龚兵 , 沈玉梅 . 酸敏感连接单元的合成及其在两亲性嵌段共聚物中的应用[J]. 有机化学, 2014 , 34(9) : 1806 -1815 . DOI: 10.6023/cjoc201403029
A series of acid cleavable linkers based on tetrahydropyran/tetrahydrofuran (THP/THF) ether were synthesized, in which both of the two terminals are active group which is easy to react with other compounds. The structures for these linkers 1a and 1b were confirmed by 1H NMR, 13C NMR, HRMS, and IR. Diblock cooligomerModel compound containing THP linkage was synthesized. Furthermore a pH-responsive amphiphilic diblock copolymer containing tetrahydrofuran (THF) linkage in which hydrophilic segment was poly(ethylene glycol) (PEG) and hydrophobic segment was hydrophobic polylactic acid (PLA) was synthesized and characterized by 1H NMR, IR and gel permeation chromatography (GPC). The size, stability and critical micelle concentration (CMC) of diblock copolymer micelle were also evaluated. The preliminary experimental results show that the acid sensitive amphiphilic diblock copolymer micelle is a potential alternative for future drug delivery.

[1] Murthy, N.; Thng, Y.X.; Schuck, S.M.; Xu, C.J.; Fréchet, M.J.J.Am.Chem.Soc.2002, 124, 12398.
[2] Barton, V.; S.Ward, A.; Chadwick, J.; Hill, A.; O'Neill, P.M.J.Med.Chem.2010, 53, 4555.
[3] Olson, E.S.; Jiang, T.; Aguilera, T.A.; Nguyen, Q.T.; Ellies, L.G.; Scadeng, M.; Tsien, R.Y.Proc.Natl.Acad.Sci.U.S.A.2010, 107, 4311.
[4] Louie, A.Y.; Hüber, M.M.; Ahrens, E.T.; Rothbächer, U.; Moats, R.; Jacobs, R.E.; Fraser, S.E.; Meade, T.J.Nat.Biotechnol.2000, 18, 321.
[5] Knapp, D.C.; Serva, S.; D'Onofrio, J.; Keller, A.; Lubys, A.; Kurg, A.; Remm, M.; Engels, J.W.Chem.Eur.J.2011, 17, 2903.
[6] Mura, S, Nicolas, J, Couvreur, P.Nat.Mater.2013, 12, 991.
[7] Cai, X.B.; Hu, W.; Lu, W.Chin.J.Org.Chem.2013, 33, 2520 (in Chinese).(蔡晓冰, 胡薇, 陆国林, 有机化学, 2013, 33, 2520.)
[8] Zhang, Q.; Re Ko, N.; Kwon Oh, J.Chem.Commun.2012, 48, 7542.
[9] Xi, C.B.; Yang, D.; Li, J.; Yan, J.J.; Hu, J.H.Chin.J.Org.Chem.2012, 32, 2166 (in Chinese).(席陈彬, 杨东, 李静, 晏建军, 胡建华, 有机化学, 2012, 32, 2166.)
[10] DiLauro, A.M.; Abbaspourrad, A.; Weitz, D.A.; Phillips, S.T.Macromolecules 2013, 46, 3309.
[11] Tannock, I.F.; Rotin, D.Cancer Res.1989, 49, 4373.
[12] Stubbs, M.; McSheehy, P.M.J.; Griffiths, J.R.; Bashford, C.L.Mol.Med.Today 2000, 6, 15.
[13] Huang, X.Macromolecules 2009, 42, 783.
[14] Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q.Macromolecules 2011, 44, 857.
[15] Jackson, A.W.; Stakes, C.; Fulton, D.A.Polym.Chem.2011, 2, 2500.
[16] Prabaharan, M.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S.Biomaterials 2009, 30, 5757.
[17] Jin, Y.; Song, L.; Su, Y.; Zhu, L.J.; Pang, Y.; Qiu, F.; Tong, G.S.; Yan, D.Y.; Zhu, B.S.; Zhu, X.Y.Biomacromolecules 2011, 12, 3460.
[18] Tanaka, H.; Ishida, T.; Matoba, N.; Tsukamoto, H.; Yamada, H.; Takahashi, T.Chem.Int.Ed.2006, 45, 6349.
[19] Klaikherd, A.; Nagamani, C.; Thayumanavan, S.J.Am.Chem.Soc.2009, 131, 4830.
[20] Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M.J.Bioconjugate Chem.2004, 15, 1254.
[21] Carceller, E.; Merlos, M.; Giral, M.; Bartroli, J.; Garcia-Rafanell, J.; Forn, J.J.Med.Chem.1992, 35, 676.
[22] Beaver, M.G.; Woerpel, K.A.J.Org.Chem.2010, 75, 1107.
[23] (a) Cai, X.; Chorghade, M.S.; Fura, A.; Grewal, G.S.; Jauregui, K.A.; Lounsbury, H.A.; Scannell, R.T.; Yeh, C.G.; Young, M.A.; Yu, S.Org.Process Res.Dev.1999, 3, 73.(b) Pilli, R.A.; Victor, M.M.; de Meijere, A.J.Org.Chem.2000, 65, 5910.(c) Shiro, Y.; Kato, K.; Fujii, M.; Ida, Y.; Akita, H.Tetrahedron 2006, 62, 8687.(d) Hamamoto, H.; Suzuki, Y.; Takahashi, H.; Ikegami, S.Adv.Synth.Catal.2007, 349, 2685.(e) Montagnat, O.D.; Lessene, G.; Hughes, A.B.J.Org.Chem.2009, 75, 390.
[24] Dixon, D.J.; Ley, S.V.; Reynolds, D.J.Chem.Eur.J.2002, 8, 1621.
[25] Nguyen-Ba, N.; Quimpere, M.; Kong, L.C.C.; Brown, W.L.; Dionne, G.US 5789394, 1998 [Chem.Abstr.1998, 129, 161816].
[26] Chow, E.K.-H.; Ho, D.Sci Transl Med 2013, 5(216), 216rv4; Schmalenberg, K.E.; Frauchiger, L.; Nikkhouy-Albers, L.; Uhrich, K.E.Biomacromolecules 2001, 2, 851.
[27] Wang, D.L.; Su, Y.; Zhu, B.S.; Pang, Y.; Zhu, L.J.; Liu, J.Y.; Tu, C.L.; Yan, D.Y.; Zhu, X.Y.Biomacromolecules 2011, 12, 1370.
/
| 〈 |
|
〉 |