

甾体肟醚化合物的合成及抗肿瘤活性研究
收稿日期: 2014-03-22
修回日期: 2014-04-21
网络出版日期: 2014-05-07
基金资助
广西自然科学基金(No. 2010GXNSFD013019)和广西教育厅重点基金(No. 201202ZD059)资助项目.
Synthesis and Antiproliferative Evaluation of Some Steroidal Oxime Ether
Received date: 2014-03-22
Revised date: 2014-04-21
Online published: 2014-05-07
Supported by
Project supported by the Natural Science Foundation of Guangxi Province (No. 2010GXNSFD013019) and the Natural Science Fund of Education Department of Guangxi Province (No. 201202ZD059).
肟醚类化合物往往表现出很好的杀虫、除草、抗菌、抗病毒以及抗肿瘤等生理活性,因而受到人们的广泛关注.从胆甾醇、豆甾醇出发,通过不同的合成路线,制备得到系列3-取代、6-取代及22-取代甲氧基肟醚和苄氧基肟醚甾体化合物,通过IR、NMR及MS等现代分析方法对合成物进行了结构表征. 同时,分别采用人胃癌细胞(SGC-7901)、人肝癌细胞(Bel-7404)和鼻咽癌细胞(CNE-2)对合成产物及部分合成中间体进行了体外抑制肿瘤细胞生长增殖活性研究. 结果表明,某些具有22-取代结构的甲氧肟醚及苄氧肟醚甾体化合物对这些肿瘤细胞株表现出较好的抑制活性,其中22-降-3β-羟基-22-苄氧肟基-豆甾-6-缩胺硫腙(15)对人鼻咽癌细胞CNE-2的IC50值为5.7 μmol/L.
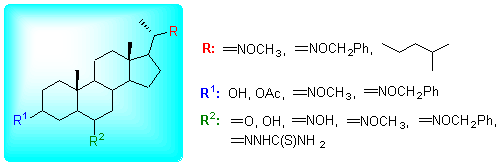
黄燕敏 , 苏绍烊 , 贾琳怡 , 甘春芳 , 林啟福 , 孔二斌 , 崔建国 . 甾体肟醚化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2014 , 34(9) : 1816 -1828 . DOI: 10.6023/cjoc201403047
Oxime ethers display remarkable insecticidal, weeding, antibacterial, antiviral and anticancer physiological activities. Series of steroidal oxime ethers with the structure of 3-, 6- or 22-O-methyloxime or O-benzyloxime were designed and synthesized by different synthetic route using cholesterol or stigmasterol as starting materials. Their structures were characterized by IR, NMR and MS. Antiproliferative activities of the compounds were assayed by thiazolyl blue tetrazolium bromide (MTT) method. Some compounds displayed distinct cytotoxicity against SGC-7901, Bel-7404 and CNE-2 cancer cell lines. Our results reveal that the compounds with the structure of 22-O-methyloxime and 22-O-benzyloxime have better antiproliferative activities. 3β-Hydroxy-22-O-benzyloxime-22,23-secostigmastan-6-thiosemicarbazone (15) displays an excellent antiproliferative activity against CNE-2 cancer cells owning an IC50 value of 5.7 μmol/L.
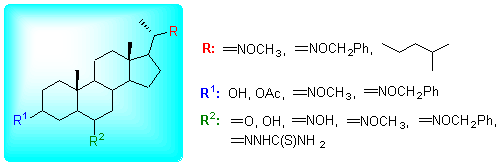
[1] Gobbini, M.; Armaroli, S.; Banfi, L.; Benicchio, A.; Carzana, G.; Fedrizzi, G.; Ferrari, P.; Giacalone, G.; Giubileo, M.; Marazzi, G.; Micheletti, R.; Moro, B.; Pozzi, M.; Scotti, P.E.; Torri, M.; Cerri, A.J.Med.Chem.2008, 51, 4601.
[2] Cushman, M.; He, H.-M.; Katzenellenbogen, J.A.; Varma, R.K.; Hamel, E.; Lin, C.M.; Ram, S.; Sachdeva, Y.P.J.Med.Chem.1997, 40, 2323.
[3] Huang, Y.-M.; Cui, J.-G.; Zhong, Z.-G.; Gan, C.-F.; Zhang, W.-Y.; Song, H.-C.Bioorg.Med.Chem.Lett.2011, 21, 3641.
[4] Shi, J.-F.; Xu, F.; Liao Q.-J.J.China Pharm.Univ.1987, 18, 169 (in Chinese).(施菊芳, 徐芳, 廖清江, 中国药科大学学报, 1987, 18, 169.)
[5] Khana, S.A.; Asiri, A.M.; Saleem, K.J.Saudi Chem.Soc.2012, 16, 7.
[6] Fan, L.; Cui, J.-G.; Wei, Y.-L.; Huang, Y.-M.Mod.Agrochem.2008, 7, 6 (in Chinese).(范磊, 崔建国, 韦英亮, 黄燕敏, 现代农药, 2008, 7, 6.)
[7] Jindal, D.P.; Chattopadhaya, R.; Guleria, S.; Gupta, R.Eur.J.Med.Chem.2003, 38, 1025.
[8] Lee, J.; Zylka, M.J.; Anderson, D.J.; Burdette, J.E.; Woodruff, T.K.; Meade, T.J.J.Am.Chem.Soc.2005, 127, 13164.
[9] Khan, S.A.; Asiri, A.M.; Saleem, K.J.Saudi Chem.Soc.2012, 16, 7.
[10] Wu, X.-D.; Liu, D.-Z.; Zhou, X.-Q.; Yang, X.-W.; Zhu, K.-M.Acta Chim.Sinica.2009, 67, 1487(in Chinese).(吴学丹, 刘东志, 周雪琴, 杨雄文, 朱孔明, 化学学报, 2009, 67, 1487.)
[11] Cerny, I.; Pouzar, V.; Hill, M.; Havlikova, H.; Hampl, R.Steroids 2006, 71, 120.
[12] Morris, K.D.W.; Amin, J.Mol.Pharmacol.2004, 66, 56.
[13] Cui, J.-G.; Huang, L.-L.; Fan, L.; Zhou, A.-M.Steroids 2008, 73, 252.
[14] Cui, J.-G.; Fan, L.; Huang, L.-L.; Liu, H.-L.; Zhou, A.-M.Steroids 2009, 74, 62.
[15] Cui, J.-G.; Fan, L.; Huang, Y.-M.; Xin, Y.; Zhou, A.-M.Steroids 2009, 74, 989.
[16] Takatsuto, S.; Ikekewa, S.N.J.Chem.Soc., Perkin Trans.1 1984, 439.
/
| 〈 |
|
〉 |