

铜催化芳硼酸与亚磷酸酯在空气下的有氧碳-磷氧化偶联反应
收稿日期: 2014-03-23
修回日期: 2014-05-10
网络出版日期: 2014-05-23
基金资助
国家自然科学基金(No.20902070)和浙江省自然科学杰出青年基金(No.LR14B020002)资助项目.
Copper-Catalyzed Aerobic Oxidative C-P Coupling of Arylboronic Acids and Diethyl Phosphite under Air
Received date: 2014-03-23
Revised date: 2014-05-10
Online published: 2014-05-23
Supported by
Project supported by the National Natural Science Foundation of China (No. 20902070) and the Natural Science Foundation of Zhejiang Province for Distinguished Young Scholars (No. LR14B020002).
徐清 , 贾小娟 , 李晓慧 , 孙清 , 周永波 , 尹双凤 , 韩立彪 . 铜催化芳硼酸与亚磷酸酯在空气下的有氧碳-磷氧化偶联反应[J]. 有机化学, 2014 , 34(7) : 1340 -1346 . DOI: 10.6023/cjoc201403048
By using Cu(OAc)2/TMEDA as the catalyst and air as the direct oxidant, arylboronic acids and diethyl phosphite can readily undergo an aerobic oxidative C-P coupling reaction under air at room temperature to give the useful arylphosphonates. This reaction uses relatively cheaper and less toxic copper salts as the catalyst, tolerates moisture and aerobic conditions. is suitable for a comparatively broad scope of substrates, and can give the target arylphosphonates in high selectivities and good yields. In comparison with conventional metal-catalyzed C-P coupling reactions of aryl halides, it is a relatively mild and efficient method for the synthesis of arylphosphonates.
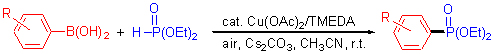
[1] (a) Quin, L. D. A Guide to Organophosphorus Chemistry, Wiley-Interscience, New York, 2000.
(b) Sasaki, M. In Chirality in Agrochemicals, Eds.: Kurihara, N.; Miyamoto, J.; Wiley & Sons, Chichester, 1998, p. 85.
(c) Imamoto, T. In Handbook of Organophosphorus Chemistry, Ed.: Engel, R., Marcel Dekker, New York, 1992, Chapter 1.
(d) Crepy, K. V. L.; Imamoto, T. Top. Curr. Chem. 2003, 229, 1.
(e) Giorgio, C.; Gianmauro, O.; Gerard, A. P. Tetrahedron 2003, 59, 9471.
(f) Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029.
[2] (a) Xu, Q.; Han, L.-B. Sci. Sinica Chim. 2010, 40, 802 (in Chinese).
(徐清, 韩立彪, 中国科学: 化学, 2010, 40, 802.)
(b) Xu, Q.; Han, L.-B. J. Organomet. Chem. 2011, 696, 130.
(c) Xu, Q.; Zhao, C.-Q.; Zhou, Y.-B.; Yin, S.-F.; Han, L.-B. Chin. J. Org. Chem. 2012, 32, 1761 (in Chinese).
(徐清, 赵长秋, 周永波, 尹双凤, 韩立彪, 有机化学, 2012, 32, 1761.)
[3] (a) Schwan, A. L. Chem. Soc. Rev. 2004, 33, 218.
(b) Glueck, D. S. Coord. Chem. Rev. 2008, 252, 2171.
(c) Prim, D.; Campagne, J.; Joseph, D.; Andrioletti, B. Tetrahedron 2002, 58, 2041.
(d) Beletskaya, I. P.; Kazankova, M. Russ. J. Org. Chem. 2002, 38, 1391.
(e) Engel, R.; Cohen, J. I. Synthesis of Carbon-Phosphorus Bonds, 2nd ed., CRC Press, Boca Raton, 2003.
[4] (a) Han, L.-B.; Tilley, T. D. J. Am. Chem. Soc. 2006, 128, 13698.
(b) Han, L.-B.; Ono, Y.; Shimada, S. J. Am. Chem. Soc. 2008, 130, 2752.
(c) Gao, Y.; Wang, G.; Chen, L.; Xu, P.; Zhao, Y.; Zhou, Y.; Han, L.-B. J. Am. Chem. Soc. 2009, 131, 7956.
(d) Zhou, Y.; Yin, S.; Gao, Y.; Zhao, Y.; Goto, M.; Han, L.-B. Angew. Chem., Int. Ed. 2010, 49, 6852.
(e) Xu, Q.; Shen, R.; Ono, Y.; Nagahata, R., Shimada, S.; Goto, M.; Han, L.-B. Chem. Commun. 2011, 47, 2333.
(f) Chen, Y.-R.; Duan, W.-L. J. Am. Chem. Soc. 2013, 135, 16754.
(g) Feng, C.-G.; Ye, M.; Xiao, K.-J.; Li, S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 9322.
(h) Unoh, Y.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2013, 52, 12975.
[5] (a) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
(b) Miyaura, N. Top. Curr. Chem. 2001, 219, 11.
(c) Candeias, N. R.; Montalbano, F.; Cal, P. M. S. D.; Gois, P. M. P. Chem. Rev. 2010, 110, 6169.
(d) Partyka, D. V. Chem. Rev. 2011, 111, 1529.
(e) Chan, D. M. T.; Lam, P. Y. S. In Boronic Acids, Ed.: Hall, D. G., Wiley-VCH, Weinheim, 2005, Chaper 5, p. 205.
(f) Xiao, J. X.; Lam, P. Y. S. Synthesis 2011, 829.
[6] (a) Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933.
(b) Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941.
(c) Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937.
[7] (a) Theil, F. Angew. Chem., Int. Ed. 1999, 38, 2345.
(b) Collman, J. P.; Zhong, M.; Zhang, C.; Costanzo, S. J. Org. Chem. 2001, 66, 7892.
(c) Rao, H.; Fu, H.; Jiang, Y.; Zhao, Y. Angew. Chem., Int. Ed. 2009, 48, 1114.
(d) Xu, H.-J.; Zhao, Y.-Q.; Feng, T.; Feng, Y.-S. J. Org. Chem. 2012, 77, 2878.
(e) Sueki, S.; Kuninobu, Y. Org. Lett. 2013, 15, 1544.
(f) Yang, M.; Pei, J.; Yan, G.; Weng, Q. Chin. J. Org. Chem. 2013, 33, 343 (in Chinese).
(杨明华, 裴吉, 严国兵, 翁秋月, 有机化学, 2013, 33, 343.)
(g) Taniguchi, N. J. Org. Chem. 2007, 72, 1241.
(h) Yu, J.-T.; Guo, H.; Yi, Y.; Fei, H.; Jiang, Y. Adv. Synth. Catal. 2014, 356, 749.
[8] Andaloussi, M.; Lindh, J.; Sävmarker, J.; Sjöerg, P. J. R.; Larhed, M. Chem. Eur. J. 2009, 15, 13069.
[9] (a) Zhuang, R.; Xu, J.; Cai, Z.; Tang, G.; Fang, M.; Zhao, Y. Org. Lett. 2011, 13, 2110.
(b) Hu, G.; Chen, W.; Fu, T.; Peng, Z.; Qiao, H.; Gao, Y.; Zhao, Y. Org. Lett. 2013, 15, 5362.
[10] (a) Ley, S. V.; Thomas, A. W. Angew. Chem., Int. Ed. 2003, 42, 5400.
(b) Evano, G.; Blanchard, N.; Toumi, M. Chem. Rev. 2008, 108, 3054.
(c) Ma, D.; Cai, Q. Acc. Chem. Res. 2008, 41, 1450.
(d) Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 6954.
[11] Allen, S. E.; Walvoord, R. R.; Padilla-Salinas, R.; Kozlowski, M. C. Chem. Rev. 2013, 113, 6234 and references therein.
[12] (a) Tian, H.; Yu, X.; Li, Q.; Wang, J.; Xu, Q. Adv. Synth. Catal. 2012, 354, 2671.
(b) Huang, B.; Tian, H.; Lin, S.; Xie, M.; Yu, X.; Xu, Q. Tetrahedron Lett. 2013, 54, 2861.
[13] During the progress of our present work, Zhao and coworkers reported similar Cu- and Ni-catalyzed C-P coupling reactions of arylboronic acids with P-H compounds (see Ref.[9]).
[14] Ouyang, K.; Xi, Z. Acta Chim. Sinica 2013, 71, 13 (in Chinese).
(欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.)
[15] (a) Kirai, N.; Yamamoto, Y. Eur. J. Org. Chem. 2009, 1864.
(b) Cheng, G.; Luo, M. Eur. J. Org. Chem. 2011, 2519.
(c) Mao, J.; Hua, Q.; Xie, G.; Yao, Z.; Shi, D. Eur. J. Org. Chem. 2009, 2262.
[16] (a) Zhao, B.; Lu, X. Tetrahedron Lett. 2006, 47, 6765.
(b) Bouffard, J.; Itami, K. Org. Lett. 2009, 11, 4410.
(c) Wong, Y.-C.; Parthasarathy, K.; Cheng, C.-H. Org. Lett. 2010, 12, 1736.
(d) Liao, Y.-X.; Xing, C.-H.; Hu, Q.-S. Org. Lett. 2012, 14, 1544.
[17] Luo, Y.; Wu, J. Organometallics 2009, 28, 6823.
[18] Ma, M.; Peng, Z.; Chen, L. Chin. J. Chem. 2006, 24, 1391.
[19] Bonnaventure, I.; Charette, A. B. J. Org. Chem. 2008, 73, 6330.
[20] Kagayama, T.; Nakano, A.; Sakaguchi, S. Org. Lett. 2006, 8, 407.
[21] Kalek, M.; Jezowska, M.; Stawinski, J. Adv. Synth. Catal. 2009, 351, 3207.
[22] Berrino, R.; Cacchi, S.; Fabrizi, G. Org. Biomol. Chem. 2010, 8, 4518.
[23] Bennett, J. A.; Hope, E. G.; Singh, K.; Stuart, A. M. J. Fluorine Chem. 2009, 130, 615.
[24] Kohler, M. C.; Sokol, J. G.; Stockland, R. A. Tetrahedron Lett. 2009, 50, 457.
/
| 〈 |
|
〉 |