

碳正离子重排反应合成Cyclocitrinol核心骨架(约稿)
收稿日期: 2014-04-17
修回日期: 2014-05-13
网络出版日期: 2014-05-23
基金资助
国家自然科学青年科学基金(No. 20902098)资助项目.
Synthesis of Cyclocitrinol Skeleton via a Carbocation Rearrangement
Received date: 2014-04-17
Revised date: 2014-05-13
Online published: 2014-05-23
Supported by
Project supported by the National Natural Science Foundation for Young Scientists of China (No. 20902098).
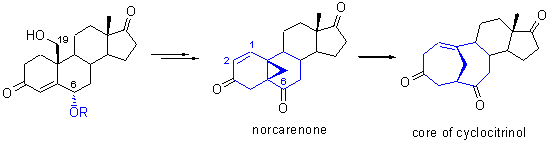
Cyclocitrinol是一类具有独特的桥环结构的天然产物,其桥环核心骨架可由高张力的降蒈烯酮开环制备得到. 以商品化的19-羟基雄甾为原料,通过高烯丙基碳正离子重排反应,高效地合成了降蒈烯酮关键中间体,完成了Cyclocitrinols天然产物的核心骨架的合成.
关键词: 甾体; Mitsunobu反应; Cyclocitrinol; 碳正离子重排
刘涛 , 严语波 , 马海燕 , 丁凯 . 碳正离子重排反应合成Cyclocitrinol核心骨架(约稿)[J]. 有机化学, 2014 , 34(9) : 1793 -1799 . DOI: 10.6023/cjoc201404033
Cyclocitrinol featured a unique bridged bicyclic skeletons, which may be constructed via SmI2-mediated reaction from a highly strained norcarenone. Herein, using commercially available 19-hydroxy-androstane as starting material, the norcarenone intermediate was synthesized via a novel homoallylic carbocation rearrangement, which was transformed into the core of cyclocitrinols.

Key words: steroids; mitsunobu reaction; cyclocitrinol; carbocation rearrangement
[1] Du, L.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M.J.Nat.Prod.2008, 71, 1343.
[2] Amagata, T.; Amagata, A.; Tenney, K.; Valeriote, F.A.; Lobkovsky, E.; Clardy, J.; Crews, P.Org.Lett.2003, 5, 4393.
[3] Kozlovsky, A.G.; Zhelifonova, V.P.; Ozerskaya, S.M.; Vinokurova, N.G.; Adanin, V.M.; Grafe, U.Pharmazie 2000, 55, 470.
[4] Marinho, A.M.D.; Rodrigues, E.; Ferreira, A.G.; Santos, L.S.J.Braz.Chem.Soc.2005, 16(6B), 1342.
[5] Aronica, S.M.; Katzenellenbogen, B.S.Mol.Endocrinol.1993, 7, 743.
[6] Sheikh, S.E.; Greffen, A.M.; Lex, J.; Neudörfl, J.M.; Schmalz, H.G.Synlett 2007, 1881.
[7] Chen, L.; Ding, K.; Tian, W.S.Chem.Commun.2003, (7), 838.
[8] Nagaoka, M.; Numazawa, M.Chem.Pharm.Bull.2004, 52, 983.
[9] Yan, Y.B; Liu, T.; Ding, K.; Tian, W.S.Chin.J.Chem.2013, 31, 63.
[10] Persky, R.; Albeck, A.J.Org.Chem.2000, 65, 3775.
[11] Diaz, S.; Gonzalez, A.; Bradshaw, B.; Cuesta, J.J.Org.Chem.2005, 70, 3749.
[12] Wang, G.J.; Martin, M.S.; Yang, H.; Williams, K.Org.Lett.2008, 10, 4203.
[13] Zhang, Q.W.; Zhou, H.Y.; You, Q.D.Chin.J.Pharm.2007, 38, 733 (in Chinese).(张庆文, 周后元, 尤启冬, 中国医药工业杂志, 2007, 38, 733.)
[14] Czemecki, S.; Valery, J.M.Synthesis 1991, (3), 239.
[15] Zhang, W.; Luo, S.; Fang, F.J.Am.Chem.Soc.2005, 127, 18.
[16] Rodebaugh, R.; Debenham, J.S.; Bert, F.R.Tetrahedron Lett.1996, 37, 5477.
[17] Ueberwasser, H.; Kalvoda, J.; Heusler, K.; Anner, G.; Wettstein, A.Helv.Chim.Acta 1963, 46, 344.
/
| 〈 |
|
〉 |