

室温下铜催化制备含羰基或酯基的喹唑啉-4(3H)-亚基类衍生物
收稿日期: 2014-02-08
修回日期: 2014-04-28
网络出版日期: 2014-06-03
基金资助
国家自然科学基金(No. 21272266)和国家“十二五”科技支撑计划课题(No. 2011BAE06B05-5)资助项目.
Copper Catalyzed Synthesis of Quinazolin-4(3H)-ylidene Derivatives Containing Carbonyl and Ester Group at Room Temperature
Received date: 2014-02-08
Revised date: 2014-04-28
Online published: 2014-06-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272266) and the National "Twelfth Five-Year" Plan for Science & Technology (No. 2011BAE06B05-5).
谢瑞龙 , 杨新玲 , 秦耀果 , 谭晓庆 , 凌云 . 室温下铜催化制备含羰基或酯基的喹唑啉-4(3H)-亚基类衍生物[J]. 有机化学, 2014 , 34(10) : 2076 -2082 . DOI: 10.6023/cjoc201402009
At room temperature, a new simple and efficient copper-catalyzed method for the synthesis of quinazolin- 4(3H)-ylidene derivatives containing carbonyl and ester group was developed. The targets were prepared using methyl 3-(2-bromophenyl)-3-oxopropanoate analogues and amidine hydrochlorides as the initial materials, cuprous iodide as catalyst, and potassium phosphate as base at the ligand-free condition. Their structures were confirmed by 1H NMR, 13C NMR and HRMS.
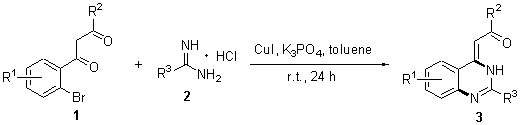
Key words: copper; room temperature; catalyst; quinazoline
[1] Cheng, Y.-J.; Sun, L.-P. Chin. J. Org. Chem. 2013, 33, 877 (in Chinese).
(成宜娟, 孙丽萍, 有机化学, 2013, 33, 877.)
[2] Rao, H.-H.; Fu, H. Synlett 2011, 745.
[3] He, T.; Ge, Y.-C., Wu, L.-L.; Fu, H.-Y.; Chen, H.; Li, X.-J. Chin. J. Catal. 2011, 32, 1376 (in Chinese).
(何亭, 葛轶岑, 武垒垒, 付海燕, 陈华, 李贤均, 催化学报, 2011, 32, 1376.)
[4] Li, Z.-K.; Wu, Z.-Q.; Deng, H.; Zhou, X.-G. Chin. J. Org. Chem. 2013, 33, 760 (in Chinese).
(李正凯, 吴之清, 邓杭, 周向葛, 有机化学, 2013, 33, 760.)
[5] Xu, H.-J.; Man. Q.-S.; Lin, Y.-C.; Li. Y.-Y.; Feng, Y.-S. Chin. J. Org. Chem. 2010, 30, 9 (in Chinese).
(许华建, 蔄秋石, 林义成, 李源源, 冯乙巳, 有机化学, 2010, 30, 9.)
[6] (a) Evano, G.; Blanchard, N.; Toumi, M. Chem. Rev. 2008, 108, 3054.
(b) Truong, V.-L.; Morrow, M. Tetrahedron Lett. 2010, 51, 758.
[7] Liu, X.-W.; Fu, H.; Jiang, Y.-Y.; Zhao, Y.-F. Angew. Chem., Int. Ed. 2009, 48, 348.
[8] Diao, X.-Q.; Wang, Y.-J.; Jiang, Y.-W.; Ma, D.-W. J. Org. Chem. 2009, 74, 7974.
[9] (a) Lv, Y.-H.; Li, Y.; Xiong, T.; Pu, W.-Y.; Zhang, H.-W.; Sun, K.; Liu, Q.; Zhang, Q. Chem. Commun. 2013, 49, 6439.
(b) Gao, Y.-L.; Xiong, Q.-Z.; An, R.; Bao, X.-P. Chin. J. Org. Chem. 2011, 31, 1529 (in Chinese).
(高元磊, 熊启中, 安锐, 鲍小平, 有机化学, 2011, 31, 1529.)
[10] Kim, Y.; Li, Z.-M.; Apetri, M.; Luo, B.-B.; Settleman, J.-E.; Anderson, K.-S. Biochemistry 2012, 51, 5212.
[11] Cao, S.-L.; Feng, Y.-P.; Jiang, Y.-Y.; Liu, S.-Y.; Ding, G.-Y.; Li, R.-T. Bioorg. Med. Chem. Lett. 2005, 15, 1915.
[12] Bianco, A.; Reghellin, V.; Donnici, L.; Fenu, S.; Alvarez, R.; Baruffa, C.; Peri, F.; Pagani, M.; Abrignani, S.; Neddermann, P.; De Francesco, R. PLoS Pathog. 2012, 8, e1002576.
[13] Lamberth, C.; Hillesheim, E.; Bassand, D.; Schaub, F. Pest Manage. Sci. 2000, 56, 94.
[14] (a) Ding, M.-W.; Yang, S.-J.; Chen Y.-F. Chin. J. Org. Chem. 2004, 24, 923 (in Chinese).
(丁明武, 杨尚君, 陈云峰, 有机化学, 2004, 24, 923.)
(b) Liu, G.; Song, B.-A.; Sang, W.-J.; Yang, S.; Jin, L.-H.; Ding, X. Chin. J. Org. Chem. 2004, 24, 1296 (in Chinese).
(刘刚, 宋宝安, 桑维钧, 杨松, 金林红, 丁雄, 有机化学, 2004, 24, 1296.)
[15] Chattopadhyay, S.-K.; Dey, R.; Biswas, S. Synthesis 2005, 1083.
[16] Molina, P.; Arques, A.; Cartagena, I.; Valcarcel, M. V. J. Heterocycl. Chem. 1985, 22, 1189.
[17] Xu, H.; Li, S.-F.; Liu, H.-X.; Fu, H.; Jiang. Y.-Y. Chem. Commun. 2010, 46, 7617.
[18] Zhao, J.; Zhao, Y.-F.; Fu, H. Angew. Chem., Int. Ed. 2011, 50, 3769.
/
| 〈 |
|
〉 |