

超临界二氧化碳介质中端炔C—H键与CO2的羧化反应研究
收稿日期: 2014-03-29
修回日期: 2014-05-16
网络出版日期: 2014-06-03
基金资助
国家自然科学基金(Nos. 21266019,21062011,21362019)资助项目.
A Study on C—H Carboxylation Reaction of Terminal Alkynes with CO2 in Supercritical CO2
Received date: 2014-03-29
Revised date: 2014-05-16
Online published: 2014-06-03
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21266019, 21062011, 21362019).
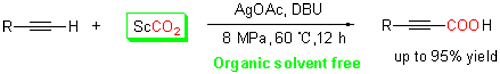
建立了一个不使用有机溶剂的端炔C—H键羧化新体系:在Ag(I)/DBU(1,8-二氮杂二环十一碳-7-烯)催化体系中,通过超临界二氧化碳与端基炔的羧化反应,以较高产率得到了相应丙炔酸产物. 在反应体系中,超临界二氧化碳既是反应物又是反应溶剂;助剂DBU起到了碱、亲核体和共催化剂的作用,并能够明显加快反应速率. 筛选出的催化体系具有良好的催化反应活性和底物适应能力,能够实现液态、固态端基炔与超临界二氧化碳的直接羧化反应,为制备功能化丙炔酸类化合物提供了一个环境友好、简单、经济的合成路线.
关键词: 醋酸银; 1,8-二氮杂二环十一碳-7-烯; 端炔; 超临界二氧化碳; 羧化反应
李发旺 , 索全伶 , 洪海龙 , 竺宁 , 王亚琦 , 韩利民 . 超临界二氧化碳介质中端炔C—H键与CO2的羧化反应研究[J]. 有机化学, 2014 , 34(10) : 2172 -2177 . DOI: 10.6023/cjoc201403064
A organic solvent free, new carboxylation pathway for C—H bond of terminal alkynes with supercritical CO2 (ScCO2) has been developed by using Ag(I)/DBU (1,8-diazabicyclo(5.4.0)undec-7-ene) catalytic system to obtain propiolic acid products with excellent yields in this work. In our reaction system, ScCO2 not only acts as reactive solvent but also as reactant. DBU plays the roles of co-catalyst, nucleophile and base, and obviously enhances the reaction rate in ScCO2. The Ag(I)/DBU catalytic system exhibits higher activity and wide substrate scope. Notably, both liquid and solid terminal alkynes can smoothly react with ScCO2 to produce desired product. The reaction pathway of functionalized propiolic acid formation from the carboxylation of terminal alkynes with CO2 is environment-friendly, simple, and economic.

Key words: AgOAc; DBU; terminal alkyne; ScCO2; carboxylation
[1] (a) Lehmann, F.; Lake, L.; Currier, E. A.; Olsson, R.; Hacksell, U.; Luthman K. Eur. J. Med. Chem. 2007, 42, 276.
(b) Ho, Y.-S.; Duh, J.-S.; Jeng, J.-H.; Wang, Y.-J.; Liang, Y.-C.; Lin, C.-H.; Tseng, C.-J.; Yu, C.-F.; Chen, R.-J.; Lin, J.-K. Int. J. Cancer 2001, 91, 393.
(c) Dong, Y.; Guo, X.-Y.; Yu, Y.-Y.; Liu, G. Mol. Diversity 2013, 17, 1.
(d) Jiang, Y.-B.; Han, C.-M.; Liang, X.-Q.; Yang, P.; Wang, H. Chin. J. Org. Chem. 2012, 32, 1884 (in Chinese).
(江玉波, 韩春美, 梁雪秋, 杨朋, 王红, 有机化学, 2012, 32, 1884.)
[2] (a) Moon, J.; Jeong, M.; Nam, H.; Ju, J.; Moon, J. H.; Jung, H. M.; Lee, S. Org. Lett. 2008, 10, 945.
(b) Li, Y.; Jardine, K. J.; Tan, R.; Song, D.; Dong, V. M. Angew. Chem., Int. Ed. 2009, 121, 9870.
(c) Bararjanian, M.; Balalaie, S.; Rominger, F.; Movassagh, B.; Bijanzadeh, H. R. J. Org. Chem. 2010, 75, 2806.
(d) Feng, H.; Ermolat'ev, D. S.; Song, G.; Van der Eycken, E. V. Adv. Synth. Catal. 2012, 354, 505.
(e) He, Z.-B.; Zhang, R.; Hu, M.-Y.; Li, L.-C.; Ni, C.-F.; Hu, J.-B. Chem. Sci. 2013, 4, 3478.
[3] Arndt, M.; Risto, E.; Krause, T.; Gooßen, L. J. ChemCatChem 2012, 4, 484.
[4] (a) Omae, I. Coord. Chem. Rev. 2012, 256, 1384.
(b) Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W. A.; Kühn, F. E. Angew. Chem., Int. Ed. 2011, 50, 8510.
(c) Tsuji, Y.; Fujihara, T. Chem. Commun. 2012, 48, 9956.
(d) Yang, Z.-Z.; He, L.-N.; Gao, J.; Liu, A.-H.; Yu, B. Energy Environ. Sci. 2012, 5, 6602.
(f) Huang, K.; Sun, C.-L.; Shi, Z.-J. Chem. Soc. Rev. 2011, 40, 2435.
[5] (a) Sakakura, T.; Choi, J.-C.; Yasuda, H. Chem. Rev. 2007, 107, 2365.
(b) Sakakura, T.; Kohon, K. Chem. Commun. 2009, 1312.
(c) Mikkelsen, M.; Jørgensena, M.; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
(d) Huang, K.; Sun, C.-L.; Shi, Z.-J. Chem. Soc. Rev. 2011, 40, 2435.
(e) Izumi, Y. Coord. Chem. Rev. 2013, 257, 171.
(f) Wang, H.-Y.; Xu, J.-F.; Jing, H.; Zhang, J.; Li, P.-Q.; Lu, F.-S. Acta Chim. Sinica 2013, 71, 941 (in Chinese).
(王祜英, 徐金凤, 井华, 张军, 李培强, 路福绥, 化学学报, 2013, 71, 941.)
[6] (a) Shi, M.; Nicholas, M. J. Am. Chem. Soc. 1997, 119, 5057.
(b) Johansson, R.; Wendt, O. F. Dalton Trans. 2007, 488.
(c) Wu, J.-G.; Hazari, N. Chem. Commun. 2011, 47, 1069.
[7] (a) Yeung, C. S.; Dong, V. M. J. Am. Chem. Soc. 2008, 130, 7826.
(b) Ochiai, H.; Jang, M.; Hirano, K.; Yorimitsu, H.; Oshima, K. Org. Lett. 2008, 10, 2681.
(c) Kobayashi, K.; Kondo, Y. Org. Lett. 2009, 11, 2035.
(d) Correa, A.; Martín, R. Angew. Chem., Int. Ed. 2009, 48, 6201.
[8] (a) Ohishi, T.; Nishiura, M.; Hou, Z. Angew. Chem., Int. Ed. 2008, 47, 5792.
(b) Takaya, J.; Tadami, S.; Ukai, K.; Iwasawa, N. Org. Lett. 2008, 10, 2697.
(c) Ukai, K.; Aoki, M.; Takaya, J.; Iwasawa. N. J. Am. Chem. Soc. 2006, 128, 8706.
(d) Zhang, X.; Zhang, W.-Z.; Shi, L.-L.; Guo, C.-X.; Zhang, L.-L.; Lu, X.-B. Chem. Commun. 2012, 48, 6292.
(e) Wang, W.-L; Zhang, G.-D.; Lang, R.; Xia, C.-G.; Li, F.-W. Green Chem. 2013, 15, 635.
[9] (a) Sasano, K.; Takaya, J.; Iwasawa, N. J. Am. Chem. Soc. 2013, 135, 10954.
(b) Zhang, L.; Hou, Z.-M. Pure Appl. Chem. 2012, 84, 1705.
(c) Ackermann. L. Angew. Chem., Int. Ed. 2011, 50, 3842.
(d) Boogaerts, I. I. F.; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858.
(e) Boogaerts, I. I. F.; Fortman, G. C.; Furst, M. R. L.; Cazin, C. S. J.; Nolan, S. P. Angew. Chem., Int. Ed. 2010, 49, 8674.
(f) Zhang, L.; Cheng, J.; Ohishi, T.; Hou. Z. Angew. Chem. Int. Ed. 2010, 49, 8670.
(g) Inomata, H.; Ogata, K.; Fukuzawa, S. I.; Hou Z. Org. Lett. 2012, 14, 3986.
(h) Vechorkin, O.; Hirt, N.; Hu, X. Org. Lett. 2010, 12, 3567.
[10] Manjolinho, F.; Arndt, M.; Gooßen, K.; Gooßen, L. J. ACS Catal. 2012, 2, 2014.
[11] (a) Yu, B.; Diao, Z.-F.; Guo, C.-X.; Zhong, C.-L.; He, L.-N.; Zhao, Y.-N.; Song, Q.-W.; Liu, A.-H.; Wang, J.-Q. Green Chem. 2013, 15, 2401.
(b) Inamoto, K.; Asano, N.; Kobayashi, K.; Yonemoto, M.; Kondo, Y. Org. Biomol. Chem. 2012, 10, 1514.
(c) Zhang, W.-Z.; Li, W.-J.; Zhang, X.; Zhou, H.; Lu, X. B. Org. Lett. 2010, 12, 4748.
(d) Oi, S.; Fukue, Y.; Nemoto, K.; Inoue, Y. Macromolecules 1996, 29, 2694.
(e) Fukue, Y.; Oi, S.; Inoue, Y. J. Chem. Soc., Chem. Commun. 1994, 2091.
[12] Gooßen, L. J.; Rodrguez, N.; Manjolinho, F.; Lange, P. P. Adv. Synth. Catal. 2010, 352, 2913.
[13] Yu, D.; Zhang, Y. Proc. Natl. Acad. Sci. U. S. A.2010, 107, 20184.
[14] Yu, D.; Tan, M. X.; Zhang, Y. Adv. Synth. Catal. 2012, 354, 969.
[15] Yu, D.; Zhang, Y. Green Chem. 2011, 13, 1275.
[16] Ouyang, K.-B.; Xi, Z.-F. Acta Chim. Sinica 2013, 71, 13 (in Chinese).
(欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.)
[17] (a) Zhang, Y.-P.; Bian, M.; Yao, W.-J.; Gu, J.-M.; Ma, C. Chem. Commun. 2009, 4729.
(b) Qi, C.; Jiang, H.; Huang, L.; Yuan, G.; Ren, Y. Org. Lett. 2011, 13, 5520.
(c) Zheng, D.-Q.; Li, S.-Y.; Wu, J. Org. Lett. 2012, 14, 2655.
(d) Li, Z.-G.; Sun, H.-B.; Jiang, H.-L.; Liu, H. Org. Lett. 2008, 10, 3263.
[18] (a) Yoshida, M.; Komatsuzaki, Y.; Ihara, M. Org. Lett. 2008, 10, 2083.
(b) Heldebrant, D. J.; Yonker, C. R.; Jessop, P. G.; Phan, L. Energy Environ. Sci. 2008, 1, 487.
(c) Dinsmore, C. J.; Mercer, S. P. Org. Lett. 2004, 6, 2885.
[19] Yoshida, M.; Mizuguchi, T.; Shishido, K. Chem. Eur. J. 2012, 18, 15578.
[20] Li, J.-H.; Jia, L.-Q.; Jiang, H.-F. Chin. J. Org. Chem. 2000, 20, 293 (in Chinese).
(李金恒, 贾兰齐, 江焕峰, 有机化学, 2000, 20, 293.)
[21] Ma, L.; Dolphin, D. J. Chem. Soc., Chem. Commun. 1995, 2251.
[22] (a) Wang, Y.; Han, Q.-Z.; Wen, H. Mol. Simul. 2013, 39, 822.
(b) Heldebrant, D. J.; Jessop, P. G.; Thomas, C. A.; Eckert, C. A.; Liotta, C. L. J. Org. Chem. 2005, 70, 5335.
(c) Endo, T.; Nagai, D.; Monma, T.; Yamaguchi, H.; Ochiai, B. Macromolecules 2004, 37, 2007.
[23] Wang, X.; Lim, Y. N.; Lee, C.; Jang, H.-Y.; Lee, B. Y. Eur. J. Org. Chem. 2013, 1867.
/
| 〈 |
|
〉 |