

六胜肽片段的液相合成及组装研究
收稿日期: 2014-03-20
修回日期: 2014-05-12
网络出版日期: 2014-06-09
基金资助
国家自然科学基金(Nos. 20872118,30070905)、陕西省重点实验室基金(Nos. 2010JS097,11JS090,12JS110)、陕西省教育厅基金(No. 08jk477)和西安市技术创新(No. CX13120)资助项目.
Synthesis of Hexapeptide by Liquid-Phase Fragments Coupling Strategy
Received date: 2014-03-20
Revised date: 2014-05-12
Online published: 2014-06-09
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20872118, 30070905), the Key Laboratory Fund of Shaanxi Province of China (Nos. 2010JS097, 11JS090, 12JS110), the Foundation of the Education Department of Shaanxi Province (No. 08jk477) and the Technology Innovation Fund of Xi'an City (No. CX13120)
张腾 , 宋卫 , 韩彬 , 刘林 , 冯凌云 , 赵金礼 , 刘建利 . 六胜肽片段的液相合成及组装研究[J]. 有机化学, 2014 , 34(10) : 2156 -2162 . DOI: 10.6023/cjoc201403043
The hexapeptide (Ac-EEMQRR-NH2) is a non-toxic cosmeceutical which can mimic the function of botulinum neurotoxins. This paper reports a solid-phase synthesis of hexapeptide by employing “A+B” fragments coupling strategy while fragment A [Ac-Glu(OtBu)-Glu(OtBu)-Met-OH] and B [H-Gln(Trt)-Arg-Arg-OH] were prepared by classical solution methods. The final product (coupling yield>65%, purity>98%) was obtained by cleavage cocktail treatment and HPLC purification. The influencing factors of coupling condition were discussed. In conclusion, this strategy is a relatively quickly, efficient and high yield method for the synthesis of hexapeptide by combining the advantages of solid phase and liquid phase synthesis.
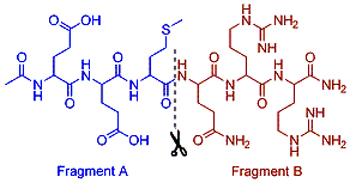
Key words: hexapeptide; peptide fragments; synthesis
[1] Merrifield, R. B. J. Am. Chem. Soc. 1936, 85, 2149.
[2] Bayer, E.; Mutter, M. Nature 1972, 237, 512.
[3] Ovchinnikov, M. V.; Bespalova, Z. D.; Molokoedov, A. S.; Revenko, I. V.; Sepetov, N. F.; Isakova, O. L.; Titov, M. I. Collect. Czech. Chem. Commun. 1989, 54, 772; 1989, 54, 784.
[4] (a) Gutiérrez, L. M.; Cànaves, J. M.; Ferrer-Montiel, A. V.; Reig, J. A.; Montal, M.; Viniegra, S. FEBS Lett. 1995, 372, 39.
(b) Ferrer-Montiel, A.V.; Gutiérrez, L. M.; Apland, J. P.; Canaves, J. M.; Gil, A.; Viniegra, S.; Biser, J.A.; Adler, M.; Montal, M. FEBS Lett. 1998, 435, 84.
(c) Blanes-Mira, C.; Merino, J. M.; Valera, E.; Fernández-Ballester, G.; Gutiérrez, L. M.; Viniegra, S.; Pérez-Payá, E.; Ferrer-Montiel, A. J. Neurochem. 2004, 88, 124.
[5] (a) Lungu, C.; Considine, E.; Zahir, S.; Ponsati, B.; Arrastia. S.; Hallett, M. Eur. J. Neurol. 2013, 20, 515.
(b) Fields, K.; Falla, T. J.; Rodan, K.; Bush, L. J. Cosmet., Dermatol. 2009, 8, 8.
(c) Lijuan, Z.; Falla, T. J. Clin. Dermatol. 2009, 27, 485.
[6] (a) Wang, Y.; Wang, M.; Xiao, X. S.; Huo, J.; Zhang, W. D. J. Cosmet. Laser Ther. 2013, 15, 237.
(b) Wang, Y.; Wang, M.; Xiao, S.; Pan, P.; Li, P.; Huo, J. Am. J. Clin. Dermatol. 2013, 14, 147.
[7] Blanes-Mira, C.; Clemente, J.; Jodas, G.; Gil, A.; Fernández-Ball- ester, G.; Ponsati, B.; Gutierrez, L.; Pérez-Payá, E.; Ferrer-Montiel, A. Int. J. Cosmet. Sci. 2002, 24, 303.
[8] (a) Jencks, W. P.; Carriuolo, J. J. Am. Chem. Soc. 1960, 82, 675.
(b) Jencks, W. P.; Gilchrist, M. J. Am. Chem. Soc. 1966, 88, 104.
[9] Zhu, L.-L.; Sheng, Z.-C.; Chen, Y.-W.; Shen, S.-B. J. Chem. Eng. Chin. Univ. 2013, 27, 791 (in Chinese).
(朱亮亮, 绳则翠, 陈英文, 沈树宝, 高校化学工程学报, 2013, 27, 791.)
[10] Mutter, M.; Maser, F. In Peptides 1982, Ed.: Blaha, K., Berlin, 1983, p. 729.
[11] Han, X.; Wang, D. X. Acta Pharm. Sin. 2007, 42, 111 (in Chinese).
(韩香, 王德心, 药学学报, 2007, 42, 111.)
/
| 〈 |
|
〉 |