

硅烷偶联化壳聚糖负载CuBr高活性催化合成1,2,3-三唑化合物
收稿日期: 2014-04-14
修回日期: 2014-05-20
网络出版日期: 2014-06-09
基金资助
国家自然科学基金(No. 21004024)、福建省自然科学基金(No. 2011J01046)、福建省“高校新世纪优秀人才支持计划”(No. 2012FJ-NCET-ZR03)和福建省“高校杰出青年科研人才培育计划”(No. 11FJPY02)以及“华侨大学中青年教师科研提升资助计划”(No. ZQN-YX103)资助项目.
Highly Efficient Synthesis of 1,2,3-Triazoles Catalyzed by Silane Coupled Chitosan-CuBr Catalyst
Received date: 2014-04-14
Revised date: 2014-05-20
Online published: 2014-06-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21004024), the Natural Science Foundation of Fujian Province (No. 2011J01046), the Program for New Century Excellent Talents in Fujian Province (No. 2012FJ-NCET-ZR03) and the University Distinguished Young Research Talent Training Program of Fujian Province (No. 11FJPY02) and the Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (No. ZQN-YX103).
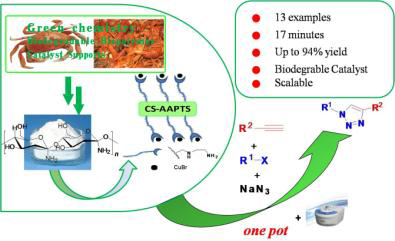
以壳聚糖为原料,将其与N-(2-氨乙基)-3-氨丙基三乙氧基硅烷(AAPTS)反应制得胺基功能化的壳聚糖(CS-AAPTS),然后将CS-AAPTS与CuBr进行络合制备得到负载型催化剂(CS-CuBr). 通过FT-IR,TGA,XRD以及EDX等分析方法对CS-CuBr催化剂进行表征. 结合微波辐射技术以及“一锅法”合成策略,以CS-CuBr为催化剂催化有机炔、卤代烷以及NaN3之间的环加成反应制备1,2,3-三唑. 结果表明,CS-CuBr具有良好的催化性能,微波辐射功率为480 W,温度为70 ℃条件下,可快速合成出1,2,3-三唑类化合物. CS-CuBr易回收和重复使用,重复使用4次后仍可保持良好收率. 初步放大实验表明,1,2,3-三唑类化合物能够以94%的收率以十数克规模制备.
江云兵 , 王彦龙 , 韩骞 , 朱荣俊 , 熊兴泉 . 硅烷偶联化壳聚糖负载CuBr高活性催化合成1,2,3-三唑化合物[J]. 有机化学, 2014 , 34(10) : 2068 -2075 . DOI: 10.6023/cjoc201404024
N-(2-Aminoethyl)-3-aminopropyltriethoxysilane-modified chitosan (CS-AAPTS) was successfully prepared by simply refluxing the corresponding CS and AAPTS in toluene solution. Subsequently, CS-AAPTS bound CuBr (CS-CuBr) was synthesized by the reaction of CS-AAPTS with CuBr in DMF at room temperature under N2 atmosphere. The obtained catalyst was characterized by FT-IR, TGA, XRD and EDX. The catalytic performances were evaluated in one-pot multicomponent copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction under microwave-assisted condition. CS-CuBr was found to exhibit obvious catalytic activity to rapidly prepare 1,2,3-triazole compounds under the microwave irradiation power of 480 W and 70 ℃ operating conditions. Furthermore, the catalyst could be easily recovered by simple filtration and recycled at least 4 cycles without significant loss of activity, and the preparation of 1,2,3-triazoles could be scaled up to multiple-gram conveniently with a yield up to 94%.

Key words: chitosan; heterogeneous catalyst; microwave-assisted; one-pot; 1,2,3-triazoles; CuAAC reaction
[1] Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem. Asian. J. 2011, 6, 2696.
[2] Bohacek, R. S.; McMartin, C.; Guida, W. C. Med. Res. Rev. 1996, 16, 3.
[3] Appukkuttan, P.; Dehaen, W.; Fokin, V. V.; Van der Eycken, E. Org. Lett. 2004, 6, 4223.
[4] Megia-Fernandez, A.; Ortega-Muñoz, M.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2012, 354, 1797.
[5] Driowya, M.; Puissant, A.; Robert, G.; Auberger, P.; Benhida, R.; Bougrin, K. Ultrason. Sonochem. 2012, 19, 1132.
[6] Xiong, X. Q.; Cai, L. Catal. Sci. Technol. 2013, 3, 1301.
[7] Kappe, C. O.; Van der Eycken, E. Chem. Soc. Rev. 2010, 39, 1280.
[8] Xiong, X. Q.; Cai, L.; Tang, Z. K. Chin. J. Org. Chem. 2012, 32, 1410 (in Chinese).
(熊兴泉, 蔡雷, 唐忠科, 有机化学, 2012, 32, 1410.)
[9] Lutz, J. F. Angew. Chem., Int. Ed. 2008, 47, 2182.
[10] Becer, C. R.; Hoogenboom, R.; Schubert, U. S. Angew. Chem., Int. Ed. 2009, 48, 4900.
[11] Dervaux, B.; Du Prez, F. E. Chem. Sci. 2012, 3, 959.
[12] Megia-Fernandez, A.; Ortega-Muñoz, M.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2010, 352, 3306.
[13] Hudson, R.; Li, C. J.; Moores, A. Green Chem. 2012, 14, 622.
[14] Baig, R. B. N.; Varma, R. S. Green Chem. 2012, 14, 625.
[15] Xiong, X. Q.; Chen, H. X.; Tang, Z. K.; Jiang, Y. B. RSC Adv. 2014, 4, 9830.
[16] Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Khosropour, A. R.; Moghadam, M.; Mirkhani, V.; Tangestaninejad, S.; Amiri Rudbari, H. J. Org. Chem. 2014, 79, 1437.
[17] Coelho, A.; Diz, P.; Caamano, O.; Sotelo, E. Adv. Synth. Catal. 2010, 352, 1179.
[18] Shamim, T.; Paul, S. Catal. Lett. 2010, 136, 260.
[19] Wan, L.; Cai, C. Catal. Lett. 2012, 142, 1134.
[20] Radatz, C. S.; do Amaral Soares, L.; Vieira, E. R.; Alves, D.; Russowsky, D.; Schneider, P. H. New J. Chem. 2014. 38, 1410.
[21] Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. J. Org. Chem. 2011, 76, 8394.
[22] Lopez Ruíz, H.; Emilio de la Cerda-Pedro, J.; Rojas-Lima, S.; Perez-Perez, I.; Viridiana Rodriguez-Sanchez, B.; Santillan, R.; Coreno, O. ARKIVOC 2013, (iii), 139.
[23] Lammens, M.; Skey, J.; Wallyn, S.; O'Reilly, R.; Du Prez, F. Chem. Commun. 2010, 46, 8719.
[24] Jalal, A.; Mosadegh, K.; Farhad, S.; Masoumeh, V. Catal. Commun. 2012, 27, 17.
[25] Roy, S.; Chatterjee, T.; Manirul Islam, S. Green Chem. 2013, 15, 2532.
[26] Xiong, X. Q.; Cai, L.; Jiang, Y. B.; Han, Q. ACS Sustainable Chem. Eng. 2014, 2, 765.
[27] Guibal, E. Prog. Polym. Sci. 2005, 30, 71.
[28] Baig, R. B. N.; Varma, R. S. Green Chem. 2013, 15, 1839.
[29] Wang, D.; Li, N.; Zhao, M. M.; Shi,W. L.; Ma, C. W.; Chen, B. H. Green Chem. 2010, 12, 2120.
[30] Doiron, J.; Soultan, A. H.; Richard, R.; Touré, M. M.; Picot, N.; Richard, R.; ?uperlovi?-Culf, M.; Robichaud, G. A.; Touaibia, M. Eur. J. Med. Chem. 2011, 46, 4010.
[31] Dilip, R. Catal. Commun. 2009, 10, 1240.
[32] Candelon, N.; Lastecoue res, D.; Diallo, A. K.; Aranzaes, J. R.; Astruc, D.; Vincent, J. M. Chem. Commun. 2008, 6, 741.
[33] Khan, S. S.; Hanelt, S., Liebscher, J. ARKIVOC 2009, (xii), 193.
[34] Iwasaki, M.; Yorimitsu, H.; Oshima, K. Chem. Asian J. 2007, 2, 1430.
/
| 〈 |
|
〉 |