

格尔德霉素C(11)~C(21)片段的合成
收稿日期: 2014-05-29
修回日期: 2014-06-05
网络出版日期: 2014-06-16
基金资助
国家自然科学基金(No. 21272279)资助项目.
Practical Synthesis of the C(11)~C(21) Fragment of Geldanamycin
Received date: 2014-05-29
Revised date: 2014-06-05
Online published: 2014-06-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272279).
李永强 , 严睿 , 卞传才 , 张智 , 刘迪 , 俞晓明 . 格尔德霉素C(11)~C(21)片段的合成[J]. 有机化学, 2014 , 34(10) : 2035 -2039 . DOI: 10.6023/cjoc201405038
The C(11)~C(21) fragment of benzoquinone ansamycin geldanamycin was synthesized in 10 steps and 24% overall yield from known lactone 5, which was prepared from tri-O-acetyl-D-glucal in 3 steps. The key steps included an α-methylenation of lactone 5, followed by a substance induced facial selective hydrogenation.
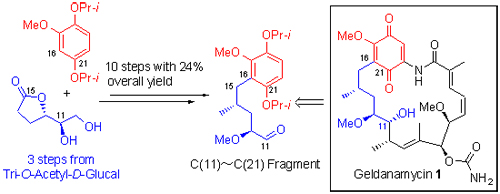
Key words: geldanamycin; chiral γ-lactone; asymmetric synthesis
[1] DeBoer, C.; Meulman, P.; Wnuk, R.; Peterson, D. J. Antibiot. 1970, 23, 442.
[2] Whitesell, L.; Mimnaugh, E. G.; De Costa, B.; Myers, C. E.; Neckers, L. M. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 8324.
[3] Garcia-Carbonero, R.; Carnero, A.; Paz-Ares, L. Lancet Oncol. 2013, 14, e358.
[4] (a) Franke, J.; Eichner, S.; Zeilinger, C.; Kirschning, A. Nat. Prod. Rep. 2013, 30, 1299.
(b) Kitson, R. R.; Moody, C. J. J. Org. Chem. 2013, 78, 5117.
(c) Kim, T.; Keum, G.; Pae, A. N. Expert Opin. Ther. Pat. 2013, 23, 919.
[5] Andrus, M. B.; Meredith, E. L.; Hicken, E. J.; Simmons, B. L.; Glancey, R. R.; Ma, W. J. Org. Chem. 2003, 68, 8162.
[6] Qin, H.-L.; Panek, J. S. Org. Lett. 2008, 10, 2477.
[7] (a) Horneff, T.; Bach, T. Synlett 2008, 2969.
(b) Hampel, T.; Neubauer, T.; van Leeuwen, T.; Bach, T. Chem.- Eur. J. 2012, 18, 10382.
[8] Bian, C.; Yan, R.; Yu, X. Tetrahedron 2014, 70, 2982.
[9] (a) Nakata, M.; Osumi, T.; Ueno, A.; Kimura, T.; Tamai, T.; Tatsuta, K. Tetrahedron Lett. 1991, 32, 6015.
(b) Canova, S.; Bellosta, V.; Bigot, A.; Mailliet, P.; Mignani, S.; Cossy, J. Org. Lett. 2007, 9, 145.
(c) Carter, K. D.; Panek, J. S. Org. Lett. 2004, 6, 55.
[10] Lee, J.; Lewin, N. E.; Acs, P.; Blumberg, P. M.; Marquez, V. E. J. Med. Chem. 1997, 40, 1560.
/
| 〈 |
|
〉 |