

3-芳基-2(5H)-呋喃酮-氟喹诺酮杂合体多靶点抗菌化合物的设计、合成及其作用机理研究(约稿)
收稿日期: 2014-04-26
修回日期: 2014-06-04
网络出版日期: 2014-07-01
基金资助
国家自然科学基金(No. 21262013)、湖南省自然科学基金(No. 13JJ2030)和湖南省高校科技创新团队支持计划资助项目.
3-Arylfuran-2(5H)-one-fluoroquinolone Hybrids as Multi-target Antibacterial Agents: Design, Synthesis and Its Mechanistic Implication
Received date: 2014-04-26
Revised date: 2014-06-04
Online published: 2014-07-01
Supported by
Project supported by the National Natural Science Foundation of China (No. 21262013), the Hunan Provincial Natural Science Foundation (No. 13JJ2030), and the Science and Technology Innovative Research Team in Higher Educational Instituions of Hunan Province.
王旭东 , 魏伟 , 邓瑞成 , 周沙沙 , 张蕾 , 林肖依 , 肖竹平 . 3-芳基-2(5H)-呋喃酮-氟喹诺酮杂合体多靶点抗菌化合物的设计、合成及其作用机理研究(约稿)[J]. 有机化学, 2014 , 34(9) : 1773 -1779 . DOI: 10.6023/cjoc201404042
Based on the multi-target drug design and hybrid strategy for drug research and development, seven new 3-arylfuran-2(5H)-one-fluoroquinolone hybrids have been designed and synthesized, in which the 3-aryl-4-(2-morpholino- ethoxy)furan-2(5H)-one (targeting tyrosyl-tRNA synthetase, TyrRS) and fluoroquinolone (targeting DNA gyrase) were merged. In the antibacterial assay, most of the tested compounds displayed a broad spectrum of activity against microorganisms including Gram-negative bacteria, Gram-positive bacteria and fungi. Furthermore, the enzyme assay and molecular docking revealed that 3-arylfuran-2(5H)-one-fluoroquinolone is a potent antibacterial agent by disturbing the processes of bacterial DNA replication and protein synthesis. These excellent results strongly suggest that 3-arylfuran-2(5H)-one-fluoroquinolone hybrid deserves to further research as a novel antibiotic.
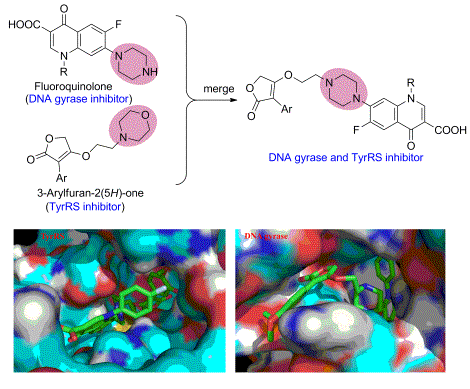
Key words: 3-arylfuran-2(5H)-one; fluoroquinolone; hybrid; multi-target; inhibitor
[1] Philip, H.EMBO Reports 2012, 13, 680.
[2] Yang, J.Q; Hu, Y.W.; Gu, Q.; Li, M.G.; Li, M.Q.; Song, B.A.Chin.J.Org.Chem.2014, 34, 829 (in Chinese).(杨家强, 胡月维, 谷晴, 李明刚, 李明强, 宋宝安, 有机化学, 2014, 34, 829.)
[3] Zhang, C.F.; Wu, X, G.; Li, Y.; Liang, C.R.; Che, Y.Z.; Gu, L.L.; Ren, J., H, K., Sun, X.Q.; Yang, C.H.; Cheng, X.Chin.J.Org.Chem.2013, 33, 1309 (in Chinese).(张成芳, 吴小刚, 李燕, 梁翠荣, 车亦舟, 顾玲玲, 任杰, 胡昆, 孙小强, Ching-Hong Yang, 陈新, 有机化学, 2013, 33, 1309.)
[4] Zhang, L.; Huang, L.Y.Chin.J.Nosocomoil.2008, 18, 897 (in Chinese).(张玲, 黄留玉, 中华医院感染学杂志, 2008, 18, 897.)
[5] Karen, B.; Michael, J.Biochem.Pharmacol.2011, 82, 1528.
[6] Nat.Rev.Microbiol.2010, 8, 836.
[7] Laxminarayan, R.; Powers, J.H.Nat.Rev.Drug Discovery 2011, 10, 727.
[8] Wang, J.Y.; Zheng, W.Chin.J.Antibiotic.2011, 36(3) 165 (in Chinese).(王娇艳, 郑卫, 中国抗生素杂志, 2011, 36(3) 165.)
[9] Aminake, M.N.; Mahajan, A.; Kumar, V.; Hans, R.; Wiesner, L.; Taylor, D.; de Kock, C.; Grobler, A.; Smith, P.J.; Kirschner, M.; Rethwilm, A.; Pradel, G.; Chibale, K.Bioorg.Med.Chem.2012, 20, 5277.
[10] Aziz, M.A.; Park, S.E.; Rahma, G.E.D.A.A.A.; Sayed, M.A.; Kwon, Y.Eur.J.Med.Chem.2013, 69, 427.
[11] Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A.; Rajtar, B.; Dacewicz, M.P.Eur.J.Med.Chem.2013, 60, 128.
[12] Garudachari, B.; Isloor, A.M.; Satyanarayana, M.N.; Fun, H.K.; Pavithra, N.; Kulal, A.Eur.J.Med.Chem.2013, 68, 422.
[13] Chai, Y.; Wan, Z.L.; Wang, B.; Guo, H.Y.; Liu, M.L.Eur.J.Med.Chem.2009, 44, 4063.
[14] Parcej, D.; Tampé, R.Nat.Chem.Biol.2010, 6, 572.
[15] Kumar, S.; Varela, M.F.Int.J.Mol.Sci.2012, 13, 4484.
[16] Poirel, L.; Bonnin, R.A.; Nordmann, P.IUBMB Life 2011, 63, 1061.
[17] Liu, G.Z.; Xu, H.W.; Sun, K.; Wang, J.; Liu, H.M.Chin.J.Org.Chem.2008, 28, 201 (in Chinese).(徐海伟, 孙凯, 王君, 刘宏民, 有机化学, 2008, 28, 201.)
[18] Xiao, Z.P.; Ouyang, H.; Wang, X.D.; Lv, P.C.; Huang, Z.J.; Yu, S.R.; Yi, T.F.; Yang, Y.L.; Zhu, H.L.Bioorg.Med.Chem.2011, 19, 3884.
[19] Xiao, Z.P.; Ma, T.W.; Liao, M.L.; Feng, Y.T.; Peng, X.C.; Li, J.L.; Li, Z.P.; Wu, Y.; Luo, Q.; Deng, Y.; Liang, X.; Zhu, H.L.Eur.J.Med.Chem.2011, 46, 4904.
[20] Xiao, Z.P.; He, X.B.; Peng, Z.Y.; Xiong, T.J.; Peng, J.; Chen, L.H.; Zhu, H.L.Bioorg.Med.Chem.2011, 19, 1571.
[21] Fostel, M.J.; Montgomery, D.A.; Shen, L.L.Antimicrob.Agents Chemother.1992, 36, 2131.
[22] Sugar, A.M.; Liu, X.P.; Chen, R.J.Antimicrob.Agents Chemother.1997, 41, 2518.
[23] Srinivasan, S.; Beema, S.R.M.; Nithyanand, P.; Manisankar, P.; Pandian, S.K.Eur.J.Med.Chem.2010, 45, 6101.
[24] Shen, L.L.; Baranowski, J.; Fostel, J.; Montgomery, D.A.; Lartey, P.A.Antimicrob.Agents Chemother.1992, 36, 2778.
[25] Park, S.G.; Schimmel, P.; Kim, S.Proc.Natl.Acad.Sci.U.S.A.2008, 105, 11043.
[26] Van de Vijver, P.; Vondenhoff, G.H.; Kazako, T.S.; Semenova, E.; Kuznedelov, K.; Metlitskaya, A.; Van Aerschot, A.; Severinov, K.J.Bacteriol.2009, 191, 6273.
/
| 〈 |
|
〉 |