

一种红光发射三苯胺吡啶盐的合成及荧光探针性质
收稿日期: 2014-03-30
修回日期: 2014-05-26
网络出版日期: 2014-07-11
基金资助
国家自然科学基金(No.61178057 )资助项目.
Systhesis and Fluorescence Probe Properities of Red-Emission Triphenylamine-Pyridinium Salt
Received date: 2014-03-30
Revised date: 2014-05-26
Online published: 2014-07-11
Supported by
Project supported by the National Natural Science Foundation of China (No.61178057).
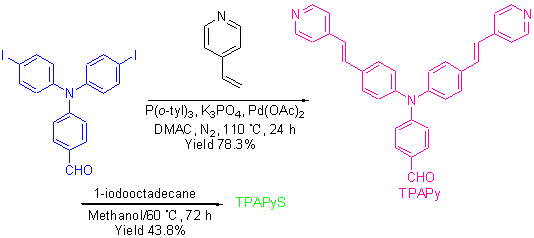
以双碘代芳醛4-[N,N-二(4-碘苯基)氨基]苯甲醛与4-乙烯基吡啶通过钯催化双位点Heck偶联反应制备了中间体TPAPy, TPAPy再与碘代十八烷反应得到吡啶盐衍生物TPAPyS. 目标化合物的结构经过红外光谱、核磁共振谱、高分辨质谱确认, 测定了吡啶盐TPAPyS在固态、水溶液中及乙醇/水混合溶液中的荧光光谱. 吡啶盐TPAPyS在固体状态下发出暗红色荧光, 荧光发射峰为 654 nm, 测得固体量子产率为3.83%. TPAPyS 在水溶液中发出红色荧光, 荧光发射峰为647 nm. 在乙醇/水混合溶液中, 化合物TPAPyS的荧光发射峰位于612~640 nm. 测定了吡啶盐TPAPyS与牛血清蛋白(BSA)、胱氨酸(DCys)及半胱氨酸(Cys)在生理条件下的光谱行为, 吡啶盐TPAPyS与BSA、氨基酸作用后荧光发射强度均增加. 吡啶盐TPAPyS是一种可溶于水的红光发射材料, 荧光发射峰位于近红外波段, 可作为荧光探针用于牛血清蛋白和氨基酸的检测.
陶在琴 , 钱鹰 . 一种红光发射三苯胺吡啶盐的合成及荧光探针性质[J]. 有机化学, 2014 , 34(11) : 2354 -2361 . DOI: 10.6023/cjoc201403065
Intermediate TPAPy was synthesised by vinyl-pyridine and 4-[N,N-bis(4-iodophenyl)amino]benzaldehyde through double-pot Heck reaction, then iodooctadecane was introduced to pyridyl forming the final pyridine salt TPAPyS. The structure of final salt was characterized by 1H NMR, 13C NMR, IR and HRMS-MALDI-TOF. Its fluorescence property was examined. Pyridine salt TPAPyS emits dark red fluorescence with peaks at 654 nm in solid state. The solid-state quantum yield of TPAPyS is 3.83%. TPAPyS emits red fluorescence with peaks at 647 nm in pure water and 612~640 nm in ethanol/H2O mixture solution. The spectra of TPAPyS with or without BSA, dicysteine, cysteine were measured. The maxima fluorescence emission intensity of water-soluble pyridine salt TPAPyS is enhanced after combining with BSA, dicysteine and cysteine. Pyridine salt TPAPyS is water-soluble with fluorescence spectra in near-infrared region, and it can be used as fluorescence probe to detect BSA, DCys and Cys.

[1] Kulinich, A. V.; Ishchenko, A. A. Chem. Rev. 2009, 78, 141.
[2] Qi, X.; Jun, E. J.; Xu, Li.; Kim, S.-J. J. Org. Chem. 2006, 71, 2881.
[3] Wu, F.-Y.; Xie, F.-Y.; Wu, Y.-M.; Hong, J.-I. J. Fluoresc. 2008, 18, 175.
[4] He, Q. W.; Miller, E. W.; Wong, A. P.; Chang, C. J. Am. Chem. Soc. 2006, 128, 9316.
[5] Tang, B.; Huang, H.; Xu, K. H.; Tong, L. L.; Yang, G. W.; Liu, X.; An, L. G. Chem. Commun. 2006, 3609.
[6] Hu, C.; Sun, W.; Cao, J. F.; Gao, P.; Wang, J. Y. Org. Lett. 2013, 15, 4022.
[7] Zhang, X. J.; Ren, X. S.; Xu, Q. H.; Chen, Z. K. Org. Lett. 2009, 11, 1257.
[8] Zhang, C. J.; Li, L.; Chen, G. Y. J.; Xu, Q. H.; Yao, S. Q. Org. Lett. 2011, 13, 4160.
[9] Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. J. Am. Chem. Soc. 2011, 133, 5680.
[10] Abnet, C. C.; Wang, Z. M.; Song, X.; Hu, N.; Zhou, F.-Y. Human Mol. Gen. 2012, 21, 9.
[11] Liu, X.; Sun, Y. M.; Zhang, Y. H.; Zhao, N. J. Fluoresc. 2011, 21, 497.
[12] Chen, Y. G.; Guo, W. H.; Ye, Z. Q.; Wang, G. L.; Yuan, J. L. Chem. Commun. 2011, 47, 6266.
[13] Kundu, K.; Knight, S. F.; Willet, N.; Lee, S. M.; Taylor, W. R.; Murthy, N. Angew. Chem., Int. Ed. 2009, 48, 299.
[14] Naama, K.-L.; Ehud, S.; Liora, O.; Moshe, P.; Ronit, S.-F.; Doron, S. J. Am. Chem. Soc. 2011, 133, 10960.
[15] Kiyose, K.; Hanaoka, K.; Oushiki, D.; Nakamura, T.; Kajimura, M. J. Am. Chem. Soc. 2010, 132, 15846.
[16] Jiang, G. X.; Susha, A. S.; Lutich, A. A.; Stefani, F. D. J. Am. Chem. Soc. 2009, 3, 4127.
[17] Chandran, S. S.; Dickson, K. A.; Raines, R. T. J. Am. Chem. Soc. 2005, 127, 1652.
[18] Jyotirmayee, M.; Nilotpal, B. J. Am. Chem. Soc. 2013, 135, 367.
[19] Lee, H.; Berezin, M. Y.; Guo, K.; Kao, J.; Achilefu, S. Org. Lett. 2009, 11, 29.
[20] Almutairi, A.; Guillaudeu, S. J.; Berezin, M. Y.; Achilefu, S. J. Am. Chem. Soc. 2008, 130, 444.
[21] Xu, C.-X.; Xu, H.-G.; Meng, R.-P.; Feng, Y.-X.; Zhang, J.-Y.; Cui, Y.-P. J. Chin. Luminesc 2006, 27, 679 (in Chinese).(徐春祥, 徐洪光, 发光学报, 2006, 27, 679.)
[22] Jin, K.-J.; Chen, X.-J.; Liu, Y.; Qin, A,-J.; Tang, B.-Z. Acta Polym. Sinica 2011, 27, 1079 (in Chinese).(金科佳, 陈秀娟, 刘一, 高分子学报, 2011, 27, 679.)
[23] Elacqua, E.; Bucar, D.-K. Org. Lett. 2009, 11, 5106.
[24] Blaise, D.; Guillaume, B.; Elodie, F.-P.; Florence, M.-B. J. Am. Chem. Soc. 2013, 135, 12697..
[25] Moineau, J.; Pozzi, G.; Quici, S.; Sinou, D. Tetrahedron Lett. 1999, 40, 7683..
[26] Yuan, C.-X.; Tao, X.-T.; Ren, Y.; Li, Y. J. Phys. Chem. C 2007, 111, 12811.
[27] Qin, P.; Liu, R.; Pan, X.; Fang, X.; Mou, Y. J. Agric. Food Chem. 2010, 58, 5561.
[28] Bhattacharya, B.; Nakka, S.; Guruprasad, L.; Samanta, A. J. Phys. Chem. B 2009, 113, 2143.
[29] Guharay, J.; Sengupta, B.; Sengupta, P. K. Proteins 2001, 43, 75.
[30] Shu, Y.; Liu, M. L.; Chen, S.; Chen, X. W. J. Phys. Chem. B 2011, 115, 12306.
[31] Ghosh, S.; Jana, S.; Guchhait, N. J. Phys. Chem. B 2012, 116, 1155.
[32] Jiang, G.-Y.; Lei, W.-H.; Zhou, Q.-X. Photochem. Photobiol. Sci. 2012, 11, 715.
[33] Maurice, M. S.; Bearne, S. L. Biochemistry 2004, 43, 2524.
[34] Shen, J.-B.; Tong, B.; Shi, J. B.; Sun, S. Acta Polym. Sinica 2010, 765 (in Chinese).(申进波, 佟斌, 石建兵, 孙书, 高分子学报, 2010, 765.)
[35] Yang, Z.-Y.; Y, T.; Chen, M.-N.; Zhang, X.-Q. Acta Polym. Sin. 2009, 560 (in Chinese).(杨志涌, 于涛, 陈美娜, 张锡奇, 高分子学报, 2009, 560.)
[36] Hong, Y. N.; Feng, C.; Yu, Y.; Liu, J.; Tang, B.-Z. Anal. Chem. 2010, 82, 7035.
[37] Luo, Y.; Huang, X.-C. Acta Chim. Sinica 2012, 70, 1295 (in Chinese).(罗懿, 黄一纯, 化学学报, 2012, 70, 7035.)
[38] Peng, G.; Liu, B.-L.; Zhao, C. X.; Jiang, Z. W. J. Graduate School Chin. Academy Sci. 2011, 28, 12.
[39] Chen, Y. H.; Yang, J. T. Biochemistry 1972, 11, 4120.
[40] Gao, H.; Lei, L. D.; Liu, J. Q. J. Photochem. Photobiol. A 2004, 167, 213..
[41] Lin, W. Y.; Long, L. L.; Yuan, L.; Cao, Z. M.; Chen, B. B. Org. Lett. 2008, 10, 5577.
[42] Madhu, S.; Gonnade, R.; Ravikanth, M. J. Org. Chem. 2013, 78, 5056.
[43] Luo, M. L.; Ying, Q. Chin. J. Org. Chem. 2012, 32, 1958 (in Chinese).(罗蔓利, 钱鹰, 有机化学, 2012, 32, 1958.)
/
| 〈 |
|
〉 |