

亲电碘环化反应合成双碘代苯并吡喃衍生物和双碘代1,2-二氢喹啉衍生物
收稿日期: 2014-02-16
修回日期: 2014-05-01
网络出版日期: 2014-08-11
基金资助
新疆维吾尔自治区自然科学基金(No.2013211A052)资助项目.
Synthesis of Bis(iodo-benzopyran) Derivatives and Bis(iodo-2H-quinoline) Derivatives by Electrophilic Cyclization
Received date: 2014-02-16
Revised date: 2014-05-01
Online published: 2014-08-11
Supported by
Project supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region of China (No.2013211A052).
罗倩 , 谢永新 , 陈朝阳 , 闫世友 , 邓文叶 , 刘义 , 王璐璐 . 亲电碘环化反应合成双碘代苯并吡喃衍生物和双碘代1,2-二氢喹啉衍生物[J]. 有机化学, 2014 , 34(12) : 2537 -2542 . DOI: 10.6023/cjoc201402019
Elecrophilic cyclization was developed from the reaction of diyne diethers and N-sulfonyl substituted anilines with ICl under mild conditions, which produced bis(iodo-benzopyran) derivatives and bis(iodo-2H-quinoline) derivatives fastly in good yields. All of above compounds have been confirmed by NMR, IR and MS analysis.
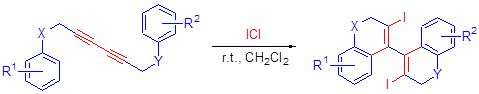
Key words: diyne diethers; electrophilic cyclization; 2H-quinoline; benzobyran
[1] (a) Aubert, C.; Buisine, O.; Malacria, M. Chem. Rev. 2002, 102, 813. (b) Wang, X. Y.; Li, Z. Z. Chin. J. Org. Chem. 2014, 34, 566 (in Chinese). (王小勇, 李治章, 有机化学, 2014, 34, 566.) (c) Rubin, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117. (d) Nakamura, I.; Yamamoto, Y. Chem. Rev. 2004. 104, 2127. (e) Kitamura, T. Eur. J. Org. Chem. 2009, 1111. (f) Yan, G. B.; Yu, J.; Zhang, L. Chin. J. Org. Chem. 2012, 32, 294 (in Chinese). (严国兵, 于健, 张玲, 有机化学, 2012, 32, 294.)
[2] Mo, J.; Eom, D.; Lee, E.; Lee, P. H. Org. Lett. 2012, 14, 3684.
[3] (a) Zhao, L. B.; Guan, Z. H.; Han, Y.; Xie, Y. X.; He, S.; Liang, Y. M. J. Org. Chem. 2007, 72, 10276. (b) Goto, K.; Yamamoto, G. Tetrahedron Lett. 2001, 42, 4875. (c) Marshall, J. A.; Yanik, M. M. J. Org. Chem. 1999, 64, 3798. (d) Kim, I.; Choi, J.; Won, H. K.; Lee, G. H. Tetrahedron Lett. 2007, 48, 6863. (e) Cherry, K.; Thibonnet, J.; Duchne, A.; Parrain, J. L. Tetrahedron Lett. 2004, 45, 2063. (f) Sakai, N.; Annaka, K.; Fujita, A.; Sato, A.; Konakahara, T. J. Org. Chem. 2008, 73, 4160. (g) Sakai, N.; Annaka, K.; Konakahara, T. Tetrahedron Lett. 2006, 631.
[4] (a) Yue, D.; Larock, R. C. J. Org. Chem. 2002, 67, 1905. (b) Hessian, K.; Flynn, B. Org. Lett. 2003, 5, 4377. (c) Larock, R. C.; Yue, D. Tetrahedron Lett. 2001, 62, 6001.
[5] (a) Arcadi, A.; Cacchi, S.; Giancarlo, F.; Marinelli, F.; Moro, L. Synlett 1999, 1432. (b) Arcadi, A.; Cacchi, S.; Giuseppe, S.; Fabrizi, G.; Marinelli, F. Org. Lett. 2002, 4, 2409. (c). Xie, Y. X.; Liu, X. Y.; Wu, L. Y.; Han, Y.; Zhao, L. B.; Fan, M. J.; Liang, Y. M. Eur. J. Org. Chem. 2008, 1013. (d) Yue, D.; Yao, T.; Larock, R. C. J. Org. Chem. 2005, 70, 10292.
[6] Knight, D. W.; Redfern, A. L.; Gilmore, J. Chem. Commun. 1998, 2207.
[7] (a) Yue, D.; Larock, R. C. Org. Lett. 2004, 6, 1905. (b) Trincado, J.; Rubio, E.; Gonzalez, J. M. Angew. Chem., Int. Ed. 2003, 42, 2406.
[8] (a) Rossi, R.; Carpita, A.; Bellina, F.; Stabile, P.; Mannina, L. Tetrahedron 2003, 59, 2067. (b) Barluenga, J.; Vazquez-Villa, H.; Balles-teros, A.; Gonzalez, J. M. J. Am. Chem. Soc. 2003, 125, 9028. (c) Peng, A. Y.; Ding, Y. X. Org. Lett. 2004, 6, 1119.
[9] Huang, Q.; Hunter, J. A.; Larock, R. C. J. Org. Chem. 2002, 67, 3437.
[10] Mo, J.; Choi, W.; Min, J.; Kim, C. E.; Eom, D.; Kim, S. H.; Lee, P. H. J. Org. Chem. 2013, 78, 11382.
[11] (a) Chen, Y.; Cho, C. H.; Larock, R. C. Org. Lett. 2009, 11, 173. (b) Du, H. A.; Tang, R. Y.; Deng, C. L.; Liu, Y.; Li, J. H. Adv. Synth. Catal. 2011, 353, 2739. (c) Du, H. A.; Zhang, X. G.; Tang, R. Y.; Li, J. H. J. Org. Chem. 2009, 74, 7844. (d) Saito, T.; Horikoshi, T.; Otani, T. Tetrahedron Lett. 2003, 44, 6513. (e) Yadav, J. S.; Reddy, B. V. S.; Rao, C. V. J. Chem. Soc. 2002, 11, 1401. (f) Luo, S. P.; Tang, R. Y.; Zhong, P.; Li, J. H. Chin. J. Org. Chem. 2009, 29, 1924 (in Chinese). (罗培松, 汤日元, 钟平, 李金恒, 有机化学, 2009, 29, 1924.) (g) Diaba, F.; Ricou, E. Bonjoch, J. Org. Lett. 2007, 9, 2633. (h) Ma, S. M.; Lu, L. H. J. Org. Chem. 2005, 70, 7629. (i) Liu, Y. H.; Zhou, S. L. Org. Lett, 2005, 7, 4609.
/
| 〈 |
|
〉 |