

发展高效的不对称Suzuki-Miyaura偶联反应及其合成应用
收稿日期: 2014-06-19
修回日期: 2014-07-22
网络出版日期: 2014-08-11
基金资助
国家自然科学基金(No. NSFC-21272254)、上海浦江人才计划(No. STCSM-13PJ1410900)、中组部青年千人计划资助项目.
Development of Efficient Asymmetric Suzuki-Miyaura Cross-Coupling and Applications in Synthesis
Received date: 2014-06-19
Revised date: 2014-07-22
Online published: 2014-08-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272254), the Science and Technology Commission of Shanghai Municipality (No. 13PJ1410900), and the “Thousand Plan” Youth Program.
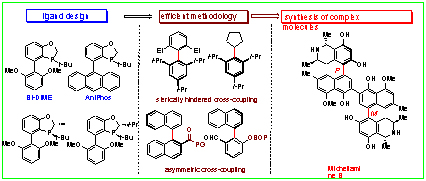
为解决不对称Suzuki-Miyaura交叉偶联反应中活性和选择性问题,我们设计并发展了一系列结构刚性的手性联芳基单膦配体. 在发展高活性的大位阻交叉偶联反应方面,成功地实现了邻位四取代芳基芳基之间的Suzuki-Miyaura交叉偶联,并发展了官能团化的大位阻交叉偶联,在邻位二取代芳基溴苯与二级烷基硼酸之间的大位阻芳基烷基交叉偶联中也取得进展. 在发展高立体选择性的交叉偶联反应方面,我们采用手性大位阻单膦配体和底物间次级作用相结合的设计理念,利用苯并噁唑啉酮辅基和芳基间的π-π作用,成功地发展了高立体选择性的邻位酰基化芳基芳基间不对称Suzuki-Miyaura偶联;利用极性基团双(2-氧代-3-噁唑烷基)次磷酰基(BOP)辅基和芳基间的极性π作用,成功地实现了应用性强的邻位氧基取代芳基芳基间高效不对称Suzuki-Miyaura偶联. 最后我们首次将高效的不对称Suzuki- Miyaura偶联方法学应用于天然产物合成,完成了手性联芳基天然产物Korupensamine A和Korupensamine B的高效不对称合成,并完成天然产物Michellamine B的立体选择性全合成.
徐广庆 , 赵庆 , 汤文军 . 发展高效的不对称Suzuki-Miyaura偶联反应及其合成应用[J]. 有机化学, 2014 , 34(10) : 1919 -1940 . DOI: 10.6023/cjoc201406030
A series of structurally rigid, sterically bulky biaryl monophosphorus ligands were designed and synthesized to investigate the reactivity and stereoselectivity of asymmetric Suzuki-Miyaura cross-coupling reaction. High efficiencies were achieved in sterically demanding cross-couplings for the synthesis of tetra-ortho-substituted biaryls with various functionalities. Sterically demanding aryl-alkyl Suzuki-Miyaura couplings between di-ortho-substituted aryl halides and secondary alkylboronic acids were also successful. In achieving efficient asymmetric Suzuki-Miyaura coupling, we implemented a new concept by employment of a chiral biaryl monophosphorus ligand developed in the group as well as utilization of a secondary interaction between two coupling partner. Using a π-π interaction of a benzooxazolidinone substituent with one aryl partner, we developed enantioselective biaryl couplings containing ortho-carbonyl substituents. High enantioselectivities were also achieved in biaryl coupling containing ortho-oxy substituents by utilizing a polar-π interaction between a polar bis(2-oxo-3-oxazolidinyl)phosphonic (BOP) substituent and one aryl partner. The methodology enabled us for the first time to implement efficient asymmetric Suzuki-Miyaura coupling in total synthesis and accomplish the syntheses of chiral biaryl natural products korupensamines A and B, ultimately a concise and stereoselective synthesis of michellamine B.

[1] (a) Bringmann, G.; Gulder, T.; Gulder, T. A. M.; Breuning, M. Chem. Rev. 2011, 111, 563.
(b) Bringmann, G.; Mortimer, A. J. P.; Keller, P. A.; Gresser, M. J.; Garner, J.; Breuning, M. Angew. Chem., Int. Ed. 2005, 44, 5384.
(c) Wang, R. W.-J.; Rebhun, L. I.; Kupchan, S. M. Cancer Res. 1977, 37, 3071.
(d) Allock, Y. F.; Manfredi, K. P.; Blunt, J. W.; Cardellina, J. H., II; Schäffer, M.; Gulden, K.-P.; Bringmann, G.; Lee, A. Y.; Clardy, J.; Francois, G.; Boyd, M. R. J. Org. Chem. 1994, 59, 6349.
(e) Boyd, M. R.; Hallock, Y. F.; Cardellina, J. H.; Manfredi, K. P.; Blunt, J. W.; McMahon, J. B.;Buckheit, R. W.; Bringmann, G.; Schäffer, M.; Cragg, G. M.; Thomas, D. W.; Jato, J. G. J. Med. Chem. 1994, 37, 1740.
[2] (a) Hayashi, T.; Hayashizaki, K.; Kiyoi, T.; Ito, Y. J. Am. Chem. Soc. 1988, 110, 8153.
(b) Genov, M.; Fuentes, B.; Espinet, P.; Pelaz, B. Tetrahedron: Asymmetry 2006, 17, 2593
[3] (a) Liu, Z.; Zhang, Y.; Wang, J. Chin. J. Org. Chem. 2013, 33, 687 (in Chinese).
(刘振兴, 张艳, 王剑波, 有机化学, 2013, 33, 687.)
(b) Xie, W.; Zuo, Z.; Zi, W.; Ma, D. J. Chin. J. Org. Chem. 2013, 33, 869 (in Chinese).
(谢卫青, 左智伟, 资伟伟, 马大为, 有机化学, 2013, 33, 869.)
[4] (a) Yin, J. J.; Buchwald, S. L. J. Am. Chem. Soc. 2000, 122, 12051.
(b) Shen, X.; Jones, G. O.; Watson, D. A.; Bhayana, B.; Buchwald, S. L. J. Am. Chem. Soc. 2010, 132, 11278.
(c) Cammidge, A. N.; Crépy, K. V. L. Chem. Commun. 2000, 1723.
(d) Cammidge, A. N.; Crépy, K. V. L. Tetrahedron 2004, 60, 4377.
(e) Bermejo, A.; Ros, A.; Fernández, R.; Lassaletta, J. M. J. Am. Chem. Soc. 2008, 130, 15798.
(f) Uozumi, Y.; Matsuura, Y.; Arakawa, T.; Yamada, Y. M. A. Angew. Chem., Int. Ed. 2009, 48, 2708.
(g) Sawai, K.; Tatumi, R.; Nakahodo, T.; Fujihara, H. Angew. Chem., Int. Ed. 2008, 47, 6917.
(h) Zhang, S.-S.; Wang, Z. Q.; Xu, M.-H.; Lin, G.-Q. Org. Lett. 2010, 12, 5546.
(i) Genov, M.; Almorin, A.; Espinet, P. Chem. Eur. J. 2006, 12, 9346.
(j) Yamamoto, T.; Akai, Y.; Nagata, Y.; Suginome, M. Angew. Chem., Int. Ed. 2011, 50, 8844.
(k) Wang, S.; Li, J.; Miao, T.; Wu, W.; Li, Q.; Zhuang, Y.; Zhou, Z.; Qiu, L. Org. Lett. 2012, 14, 1966
[5] (a) Dai, C.; Fu, G. C. J. Am. Chem. Soc. 2001, 123, 2719.
(b) Yin, J. J.; Rainka, M. P.; Zhang, X.-X.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 1162.
(c) Barder, T. F.; Walker, S. D.; Martinelli, J. R.; Buchwald, S. L. J. Am. Chem. Soc. 2005, 127, 4085.
[6] Tang, W.; Capacci, A. G.; Wei, X.; Li, W.; White, A.; Patel, N. D.; Savoie, J.; Gao, J. J.; Rodriguez, S.; Qu, B.; Haddad, N.; Lu, B. Z.; Krishnamurthy, D.; Yee, N. K.; Senanayake, C. H. Angew. Chem., Int. Ed. 2010, 49, 5879
[7] Zhao, Q.; Senanayake, C. H.; Tang, W. Chem. Eur. J. 2013, 19, 2261
[8] Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461
[9] Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2011, 111, 1417.
[10] (a) Dreher, S. P.; Dormer, P. G.; Sandrock, D. C.; Molander, G. A. J. Am. Chem. Soc. 2008, 130, 9257.
(b) Han, C.; Buchwald, S. L. J. Am. Chem. Soc. 2009, 131, 7532
[11] Li, C.; Xiao, G.; Zhao, Q.; Liu, H.; Wang, T.; Tang, W. Org. Chem. Front. 2014, 1, 225
[12] Liu, G.; Xu, G.; Luo, R.; Tang, W. Synlett 2013, 2465
[13] Tang, W.; Patel, N. D.; Xu, G.; Xu, X.; Savoie, J.; Ma, S.; Hao, M.-H.; Keshipeddy, S.; Capacci, A. G.; Wei, X.; Zhang, Y.; Gao, J. J.; Li, W.; Rodriguez, S.; Lu, B. Z.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2012, 14, 2258
[14] Xu, G.; Fu, W.; Liu, G.; Senanayake, C. H.; Tang, W. J. Am. Chem. Soc. 2014, 136, 570
[15] (a) Cozzi, F.; Cinquini, M.; Annuziata, R.; Siegel, J. S. J. Am. Chem. Soc. 1993, 115, 5330.
(b) Chen, C.-T.; Siegel, J. S. J. Am. Chem. Soc. 1994, 116, 5959.
(c) Pace, C. J.; Gao, J. Acc. Chem. Res. 2013, 46, 907
[16] Liu, G.; Liu, X.; Cai, Z.; Jiao, G.; Xu, G.; Tang, W. Angew. Chem., Int. Ed. 2013, 52, 4235.
/
| 〈 |
|
〉 |