

吡唑环桥连简约型长春碱类似物的设计合成
收稿日期: 2014-07-29
修回日期: 2014-08-27
网络出版日期: 2014-08-29
基金资助
国家自然科学基金(No.81172982)资助项目.
Design and Synthesis of Pyrazol-Bridged Simplified Vinblastine Analogues
Received date: 2014-07-29
Revised date: 2014-08-27
Online published: 2014-08-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 81172982).
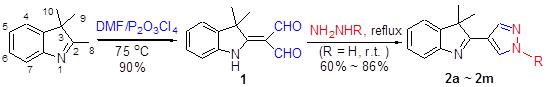
利用焦磷酰氯(P2O3Cl4)和DMF为Vilsmeier-Haack试剂, 与2,3,3-三甲基-3H-吲哚反应生成二甲酰化中间体化合物, 再与肼衍生物反应生成系列吡唑环桥连简约型长春碱类似物2a~2m. 目标化合物的结构均经1H NMR, 13C NMR和HRMS确证. 初步的抗肿瘤活性数据表明, 在50 μmol/L的浓度下, 大部分目标化合物对人乳腺癌细胞株(MCF-7) (estrogen-positive)和人肝癌细胞株(HepG2)具有一定的抗肿瘤活性, 其中2f和2j抗肿瘤作用较强, 它们对MCF-7细胞株的存活率分别为28.0%和21.4%; 而对HepG2细胞株的存活率分别为31.6%和34.0%.
国翠平 , 彭群龙 , 潘龙 , 张冬梅 , 陈河如 . 吡唑环桥连简约型长春碱类似物的设计合成[J]. 有机化学, 2014 , 34(12) : 2505 -2510 . DOI: 10.6023/cjoc201407042
A series of pyrazol-brigded simplified vinblastine analogues 2a~2m have been synthesized by the reaction of hydrazine derivatives with diformyl intermediates, which were prepared from 2,3,3-trimethyl-3H-indoles using P2O3Cl4/DMF as Vilsmeier-Haack reagent. All the target compounds have been identified by 1H NMR, 13C NMR and HRMS. The preliminary results of anticancer tests indicated that most of the compounds exhibit certain extent of anticancer activity against both MCF-7 (estrogen-positive) and HepG2 cell lines at the concentration of 50 μmol/L, respectively. 2f and 2j are the two best active compounds, where their cell viabilities against MCF-7 cell line are 28.0% and 21.4%, respectively; while against HepG2 cell line, 31.6% and 34.0%, respectively.

[1] Noble, R. L.; Beer, C. T.; Cutts, J. H. Ann. N. Y. Acad. Sci. 1958, 76, 882.
[2] Svoboda, G. H.; Neuss, N.; Gorman, M. J. J. Am. Pharm. Assoc. 1959, 48, 659.
[3] Dancey, J.; Steward, W. P. Anti-cancer Drugs 1995, 6, 625.
[4] (a) Langlois, N.; Gueritte, F.; Langlois, Y.; Potier, P. J. Am. Chem. Soc. 1976, 98, 7017. (b) Cros, S.; Wright, M.; Morimoto, M.; Lataste, H.; Couzinier, J. P.; Krikorian, A. Semin. Oncol. 1989, 16, 15.
[5] Sasaki, Y.; Kato, D.; Boger, D. L. J. Am. Chem. Soc. 2010, 132, 13533.
[6] Schleicher, K. D.; Sasaki, Y.; Tam, A. Kato, D.; Duncan, K. K.; Boger, D. L. J. Med. Chem. 2013, 56, 483.
[7] Keglevich, P.; Hazai, L.; Kalaus, G.; Szántay, C. Molecules 2012, 17, 5893.
[8] Ngo, Q. A.; Roussi, F.; Thoret, S.; Guéritte, F. Chem. Biol. Drug Des. 2010, 75, 284.
[9] Zheng, J.; Deng, L. J.; Chen, M. F.; Xiao, X. Z.; Xiao, S. W.; Guo, C. P.; Xiao, G. K.; Bai, L. L.; Ye, W. C.; Zhang, D. M.; Chen, H. R. Eur. J. Med. Chem. 2013, 65, 158.
[10] Baradarani, M. M.; Afghan, A.; Zebarjadi, F.; Hasanzadeh, K.; Joule, J. A. J. Heterocycl. Chem. 2006, 43, 1591.
[11] Rashidi, A.; Afghan, A.; Baradarani, M. M.; Joule, J. A. J. Heterocycl. Chem. 2009, 46, 428.
[12] Ye, J. H.; Ye, W. F.; Xiao, C. T.; Chen, Y.; Wang, G. P.; Zhang, W. Chin. J. Org. Chem. 2012, 32, 1503 (in Chinese). (叶家海, 叶文芳, 肖承涛, 陈 雨, 王光普, 张文超, 有机化学, 2012, 32, 1503.)
[13] Kumar, V.; Kaur, K.; Karelia, D. N.; Beniwal, V.; Gupta, G. K.; Sharma, A. K.; Gupta, A. K. Eur. J. Med. Chem. 2014, 81, 267.
[14] Zhang, D. M.; Liu, J. S.; Tang, M. K.; Liu, A.; Cao, H. H.; Jiang, L.; Chan, J. Y.; Tian, H. Y.; Fung, K. P.; Ye, W. C. Eur. J. Pharmacol. 2012, 692, 19.
[15] Gigant, B.; Wang, C. G.; Ravelli, R. B. G.; Roussi, F.; Steinmetz, M. O.; Curmi, P. A.; Sobel, A.; Knossow, M. Nature 2005, 435, 519.
/
| 〈 |
|
〉 |