

3-吗啉基氟化硼络合二吡咯甲川类化合物的合成及反应机理研究
收稿日期: 2014-07-11
修回日期: 2014-09-07
网络出版日期: 2014-09-18
基金资助
国家自然科学基金(No. 20872051)资助项目.
Synthesis and Mechanism Study of 3-Morpholin Boron- dipyrrolemethene Derivatives
Received date: 2014-07-11
Revised date: 2014-09-07
Online published: 2014-09-18
Supported by
Project supported by the National Natural Science Foundation of China (No.20872051).
贺琳彦 , 胡全子 , 席海涛 , 孙小强 . 3-吗啉基氟化硼络合二吡咯甲川类化合物的合成及反应机理研究[J]. 有机化学, 2015 , 35(1) : 232 -235 . DOI: 10.6023/cjoc201407021
Two new 3-morpholin boron-dipyrrolemethene (BODIPY) derivatives, 4,4-difluoro-8-[4'-(3-morpholinopropoxy)- phenyl]-3-morpholin-4-bora-3a,4a-diaza-s-indacene and 4,4-difluoro-8-(4-methoxyphenyl)-3-morpholin-4-bora-3a,4a-diaza- s-indacene, were designed and synthesized from p-hydroxybenzaldehyde and pyrrole. The key intermediates and the target compounds were characterized by 1H NMR and LC-MS. The mechanism of direct functionalization of BODIPY dyes at the 3-position was investigated with similarity experiments and the crystal structure.
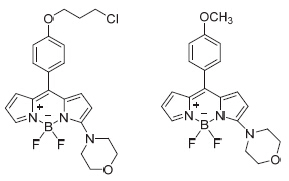
Key words: BODIPY; synthesis; reaction mechanism
[1] Loudet, A.; Burgess. K. Chem. Rev. 2007, 107, 4891.
[2] Lu, Z. T.; Zhang, X. G.; Wu, Z. M.; Zhai, T. T.; Xue, Y. A.; Mei, L.; Li, C. X. RSC Adv. 2014, 4, 19495.
[3] Zhao, T. T.; Chen, Q. Y.; Wang, P. D.; Chen Z P. RSC Adv. 2014, 4, 10390.
[4] Ozdemir, T.; Sozmen, F.; Mamur, S.; Tekinay, T.; Akkaya, E. U. Chem. Commun. 2014, 50, 5455.
[5] Mirloup, A.; Retailleau, P.; Ziessel, R. Tetrahedron Lett. 2013, 54, 4456.
[6] Imai, G.; Kogure, H.; Ogiso, A.; Misawa, T.; Nishimoto, T.; Tsukahara, H.; Takuma, K. JP 2000001509, 2000 [Chem. Abstr. 2000, 132, 64968].
[7] Mesropyan, E. G.; Ambartsumyan, G. B.; Avetisyan, A. A.; Galstyan, A. S.; Kirakosyan, A. N. Khimiya Geterotsiklicheskikh Soedinenii, 2005, (11), 1691.
[8] Dilek, Ö.; Bane, S. L. Tetrahedron Lett. 2008, 49, 1413.
[9] Dilek, Ö.; Bane, S. L. Bioorg. Med. Chem. Lett. 2009, 19(284), 6911.
[10] Imai, G.; Kogure, H.; Ogiso, A.; Misawa, T.; Nishimoto, T.; Tsukahara, H.; Takuma, K. JP 2000039716, 2000 [Chem. Abstr. 2000, 132, 144418].
[11] Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. J. Am. Chem. Soc. 2005, 127, 12162.
[12] Leen, V.; Auweraer, M. V.; Boens, N.; Dehaen, W. Org. Lett. 2011, 13(6), 1470.
[13] Leen, V.; Gonzalvo, V. Z.; Deborddraeve, W. W.; Boens, N.; Dehaen, W. Chem. Commun. 2010, 46, 4908.
[14] Verbelen, B.; Leen, V.; Wang, L. N.; Boens, N.; Dehaen, W. Chem. Commun. 2012, 48, 9129.
[15] Steiner, T. Angew. Chem., Int. Ed. 2002, 41, 48.
[16] Calhorda, M. J. Chem. Commun. 2000, 801.
[17] Littler, B. J.; Miller, M. A.; Hung, C. H.; O'Shea, D. F.; Boyle, P. D.; Lindsey, J. S. J. Org. Chem. 1999, 64, 1391.
/
| 〈 |
|
〉 |