

硼酸催化三组分无溶剂“一锅法”合成5-[(3-吲哚基)-甲基]-2,2-亚丁基-1,3-二噁烷-4,6-二酮衍生物
收稿日期: 2014-07-09
修回日期: 2014-10-10
网络出版日期: 2014-10-13
基金资助
国家科技攻关计划(No. 2001BA323C)和江西省研究生创新基金(No. YC10A51)资助项目.
Solvent-Free One-Pot Three-Component Synthesis of 5-[(Indol-3-yl)-methyl]-2,2-butylidene-1,3-dioxane-4,6-dione Derivatives with B(OH)3 as Catalyst
Received date: 2014-07-09
Revised date: 2014-10-10
Online published: 2014-10-13
Supported by
Project supported by the National Science and Technology Project (No. 2001BA323C) and the Graduate Innovation Foundation of Jiangxi Province (No. YC10A51).
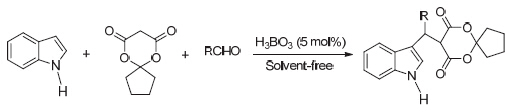
在硼酸催化下, 以吲哚、醛和2,2-亚丁基-1,3-二噁烷-4,6-二酮为原料, 经三组分无溶剂条件合成了9种5-[(3-吲哚基)-甲基]-2,2-亚丁基-1,3-二噁烷-4,6-二酮衍生物. 当催化剂的用量为5 mol%时, 60 ℃反应30~90 min, 收率为 68.6%~91.3%. 此外, 还探讨了硼酸可能的催化机理. 该方法具有反应条件温和, 反应时间短且收率高的优点. 硼酸催化剂对环境友好且可循环利用.
林春花 , 许招会 , 廖维林 , 邱曾烨 , 夏剑辉 . 硼酸催化三组分无溶剂“一锅法”合成5-[(3-吲哚基)-甲基]-2,2-亚丁基-1,3-二噁烷-4,6-二酮衍生物[J]. 有机化学, 2015 , 35(1) : 212 -216 . DOI: 10.6023/cjoc201407013
Nine kinds of 5-[(indol-3-yl)-methyl]-2,2-butylidene-1,3-dioxane-4,6-dione derivatives were synthesized by the three-component one-pot reaction of indole with aldehydes and 2,2-butylidene-1,3-dioxane-4,6-dione in the presence of boric acid under solvent-free. The results indicated that the yields ranged from 68.6% to 91.3%, when using 5 mol% boric acid and reacting at 60 ℃ for 30~90 min. Furthermore, a proposed reaction mechanism for the reaction catalyzed by boric acid was speculated. The main advantages of the present procedure were milder conditions, shorter reaction time and higher yields. Further study showed that boric acid was environmentally friendly and reused for six times without any noticeable decrease in the catalytic activity.

[1] Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796.
[2] Yang, D. L.; Li, J. R.; Sun, K. N. Chin. J. Org. Chem. 2013, 33, 2341 (in Chinese). (杨得利, 李家荣, 孙克宁, 有机化学, 2013, 33, 2341.)
[3] Yi, Z. K.; Tian, S. B. Chin. J. Org. Chem. 2014, 34, 387 (in Chinese). (伊志奎, 田拴宝, 有机化学, 2014, 34, 387.)
[4] Chen, Z. W.; Bi, J. H.; Su, W. K. Chin. J. Chem. 2013, 31, 507.
[5] Tong, G. J.; Xu, H. W.; Fan, W.; Jiang, B.; Wang, S. L.; Tu, S. J. Chin. J. Chem. 2013, 31, 1034.
[6] Francesco, E.; Salvatore, G.; Ornelio, R. Tetrahedron Lett. 2011, 52, 568.
[7] Janos, G.; Gabor, P.; Tamas, N. J. Comb. Chem. 2005, 7, 530.
[8] Marcelo, V. H.; Ingo, K.; Nayanede, S. ACS Comb. Sci. 2012, 14, 434.
[9] Janos, G.; Gyorgy, D.; Ferenc, D. QSAR Comb. Sci. 2006, 25, 439.
[10] Yuji, O.; Hitoshi, H.; Osamu, Y. Tetrahedron Lett. 1978, 20, 1759.
[11] Csaba, N.; Laurent, J.; Janos, S. Tetrahedron 2000, 56, 5479.
[12] Andrea, R.; Emmanuel, D.; Antonella, F. J. Org. Chem. 2008, 73, 6824.
[13] Srivari, C.; Vidyavathi, P.; Gangireddy, P. Tetrahedron Lett. 2012, 53, 6223.
[14] Eric, F.; Aaron, M. D.; Bryan, A. K. J. Org. Chem. 2006, 71, 409.
[15] Dumas, A. M.; Seed, A.; Zorzitto, A. K.; Fillion, E. Tetrahedron Lett. 2007, 48, 7072.
[16] Goutam, B.; Suvankar, C. RSC Adv. 2014, 4, 7380.
[17] (a) Ganguly, N. C.; Roy, S.; Mondal, P. Synth. Commun. 2014, 44, 433. (b) Halimehjnai, A. Z.; Hosseyni, S.; Gholami, H.; Hashemi, M. M. Synth. Commun. 2013, 43, 191. (c) Meshram, H. M.; Rao, N. N.; Thakur, P. B.; Reddy, B. C.; Ramesh, P. Indian J. Chem. 2013, 52, 814. (d) Nguyen, T. B.; Sorres, J.; Tran, M. Q.; Ermolenko, L.; Al-Mourabit, A. Org. Lett. 2012, 14, 3202. (e) Singh, M.; Fatma, S.; Ankit, P.; Singh, S. B.; Singh, J. Tetrahedron Lett. 2014, 55, 525.
[18] Kondaiah, G. C.; Reddy, L. A.; Babu, K. S.; Gurav, V. M.; Huge, K. G.; Bandichhor, R.; Reddy, P. P.; Bhattachhrya, A.; Anand, V. R. Tetrahedron Lett. 2008, 49, 106.
[19] Liu, L. T.; Zhang, Y.; Jiao, J.; Yang, F. Y.; Lu, H. J. Acta Chim. Sinica 2013, 71, 535 (in Chinese). (刘丽婷, 张莹, 焦竟, 杨芃原, 陆豪杰, 化学学报, 2013, 71, 535.)
[20] Zorkun, I. S.; Sarae, S.; Celebi, S. Bioorg. Med. Chem. 2006, 14, 852.
[21] Chaudhuri, M. K.; Hussain, S. J. Mol. Catal. A: Chem. 2007, 269, 214.
[22] Xu, Z. H.; Lin, C. H.; Xia, J. H. Heterocycl. Lett. 2013, 3, 319.
[23] Wang, C.; Zhang, Y. Q.; Li, G. S.; Li, J. C.; Li, X. L. Chin. J. Org. Chem. 2003, 23, 1416 (in Chinese). (王春, 张英群, 李贵深, 李敬慈, 李晓陆, 有机化学, 2003, 23, 1416.)
[24] Xu, Z. H. Chin. J. Org. Chem. 2014, 34, 1687 (in Chinese). (许招会, 有机化学, 2014, 34, 1687.)
[25] Xu, Z. H.; Li, C. H. Chin. J. Org. Chem. 2013, 33, 1540 (in Chinese). (许招会, 林春花, 有机化学, 2013, 33, 1540.)
[26] Yan. N.; Xiong, B.; Liao, W. L.; Xu, Z. H. Chin. J. Org. Chem. 2010, 30, 1391 (in Chinese). (严楠, 熊斌, 廖维林, 许招会, 有机化学, 2010, 30, 1391.)
/
| 〈 |
|
〉 |