

负载型过渡金属催化剂在碳-碳键偶联反应中的应用研究进展
收稿日期: 2014-08-13
修回日期: 2014-10-07
网络出版日期: 2014-10-16
基金资助
安徽省教育厅自然科学重点(No. KJ2013A122)资助项目.
Advances in Carbon-Carbon Coupling Reactions Catalyzed by Supported Transition-Metal Catalysts
Received date: 2014-08-13
Revised date: 2014-10-07
Online published: 2014-10-16
Supported by
Project supported by the Natural Key Project of Education Department of Anhui Province (No. KJ2013A122).
刘杰 , 朱庆仁 , 杜娟 , 张袖丽 . 负载型过渡金属催化剂在碳-碳键偶联反应中的应用研究进展[J]. 有机化学, 2015 , 35(1) : 15 -28 . DOI: 10.6023/cjoc201408014
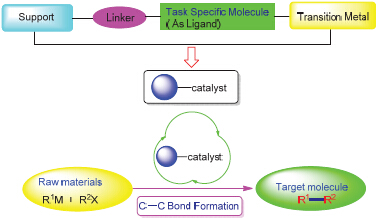
The transition-metal catalyzed coupling reaction is one of the versatile methods for the generation of new carbon- carbon bonds in organic synthesis, and it has become a hot topic in current organic chemistry. Transition metal salts and their complexes were used as catalyst directly in the classic methods with the drawbacks of high cost of the transition metal, the metal contamination of the product and the toxicity remained. In order to overcome these problems, developing green synthetic methods based on efficient and reusable catalytic systems is more desirable in organic synthesis. In this paper, the recent progress on the carbon-carbon coupling reactions catalyzed by the supported transition-metal catalysts has been reviewed.
[1] (a) Ruan, J. W.; Xiao, J.-L. Acc. Chem. Res. 2011, 44, 614. (b) Xia, Q.-H.; Ge, H.-Q.; Ye, C.-P.; Liu, Z.-M.; Su, K.-X. Chem. Rev. 2005, 105, 1603. (c) Ren, P.; Salihu, I.; Scopelliti, R.; Hu, X. Org. Lett. 2012, 14, 1748. (d) Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359.
[2] (a) Marcial, M.-M.; Roser, P. Acc. Chem. Res. 2003, 36, 638. (b) Yan, M.; Feng, X. Chin. J. Org. Chem. 2010, 30, 623 (in Chinese). (颜美, 冯秀娟, 有机化学, 2010, 30, 623.) (c) Leadbeater, N. E.; Marco, M. Chem. Rev. 2002, 102, 3217. (d) Lu, J.; Toy, P. H. Chem. Rev. 2009, 109, 815. (e) Buchmeiser, M. R. Chem. Rev. 2009, 109, 303. (f) Bergbreiter, D. E.; Tian, J.; Hongfa, C. Chem. Rev. 2009, 109, 530.
[3] Köhler, K.; Wagner, M.; Djakovitch, L. Catal. Today 2001, 66, 105.
[4] Davies, I. W.; Matty, L.; Hughes, D. L.; Reider, P. J. J. Am. Chem. Soc. 2001, 123, 10139.
[5] Iyer, S.; Thakur, V. V. J. Mol. Catal. A 2000, 157, 275.
[6] Kabalka, G. W.; Pagni, R. M.; Hair, C. M. Org. Lett. 1999, 1, 1423.
[7] Melucci, M.; Barbarella, G.; Sotgiu, G. J. Org. Chem. 2002, 67, 8877.
[8] Li, X.; Yan, X.-Y.; Chang, H.-H.; Wang, L.-C.; Zhang, Y.; Chen, W.-W.; Li, Y.-W.; Wei, W.-L. Org. Biomol. Chem. 2012, 10, 495.
[9] Marck, G.; Villiger, A.; Buchecker, R. Tetrahedron Lett. 1994, 35, 3277.
[10] Köhler, K.; Heidenreich, R. G.; Krauter, J. G. E.; Pietsch, J. Chem. Eur.-J. 2002, 8, 622.
[11] Xie, X.-G; Lu, J.-P.; Chen, B.; Han, J.-J.; She, X.-G.; Pan, X.-F. Tetrahedron Lett. 2004, 45, 809.
[12] Ambulgekar, G. V.; Bhanage, B. M.; Samant, S. D. Tetrahedron Lett. 2005, 46, 2483.
[13] Chu, W.; Li, X.; Hou, Y.; Wang, H.; Li, H.; Yuan, X.; Sun, Z. Appl. Organomet. Chem. 2012, 26, 478.
[14] Liu, C.; Rao, X.; Zhang, Y.; Li, X.; Qiu, J.; Jin, Z. Eur. J. Org. Chem. 2013, 4345.
[15] Terasawa, M.; Kaneda, K.; Imanaka, T.; Teranishi, S. J. Organomet. Chem. 1978, 162, 403.
[16] Zhang, Z. Y.; Pan, Y.; Hu, H. W.; Kao, T. Y. Synthesis 1991, 539.
[17] Schwarz, J.; Bohm, V. P. W.; Gardiner, M. G.; Grosche, M.; Herrmann, W. A.; Hieringer, W.; Raudaschl-Sieber, G. Chem. Eur.-J. 2000, 6, 1773.
[18] Lin, K. H.; Song, M. P.; Cai, D. M.; Hao, X. Q.; Wu, Y. J.; Tetrahedron Lett. 2003, 44, 3955.
[19] Yamada, Y. M. A.; Takeda, K.; Takahashi, H.; Ikegami, S.; Tetrahedron Lett. 2003, 44, 2379.
[20] (a) Li, P.; Wang, L.; Zhang, Y.; Wang, M. Tetrahedron Lett. 2008, 49, 6650. (b) Synfacts 2009, 2009(1), 0104.
[21] (a) Li, P.; Zhang, Y.; Wang L. Chem. Eur.-J. 2009, 15, 2045. (b) Zhang, Y.; Li, P.; Wang, M.; Wang, L. J. Org. Chem. 2009, 74, 4364.
[22] Houdayer, A.; Schneider, R.; Billaud, D.; Ghanbaja, J.; Lambert, J. Synth. Met. 2005, 151, 165.
[23] Yang, Y. F.; Zhou, R. X.; Zhao, S. F.; Li, Q. L.; Zheng, X. M. J. Mol. Catal. A 2003, 192, 303.
[24] Jang, S.-B. Tetrahedron Lett. 1997, 38, 1793.
[25] Li, Y.; Hong, X. M.; Collard, D. M.; El-Sayed, M. A. Org. Lett. 2000, 2, 2385.
[26] Uozumi, Y.; Nakai, Y. Org. Lett. 2002, 4, 2997.
[27] Liu, Y.; Khemtong, C.; Hu, J. Chem. Commun. 2004, 398.
[28] Wu, L.; Li, B.-L.; Huang, Y.-Y.; Zhou, H.-F.; He, Y.-M.; Fan, Q.-H. Org. Lett. 2006, 8, 3605.
[29] Yang, J.; Li, P.; Wang, L. Synthesis 2011, 1295.
[30] Razler, T. M.; Hsiao, Y.; Qian, F.; Fu, R.; Khan, R. K.; Doubleday, W. J. Org. Chem. 2009, 74, 1381.
[31] Han, J.; Liu, Y.; Guo, R. J. Am. Chem. Soc. 2009, 131, 2060.
[32] Zhou, W.-J.; Wang, K.-H.; Wang, J.-X.; Huang, D.-F. Eur. J. Org. Chem. 2010, 416.
[33] Wang, L.; Li, P.; Zhang, Y. Chem. Commun. 2004, 514.
[34] Li, P.; Wang, L.; Li, H. Tetrahedron 2005, 61, 8633.
[35] Audic, N.; Clavier, H.; Mauduit, M.; Guillemin, J. C. J. Am. Chem. Soc. 2003, 125, 9248.
[36] Corma, A.; García, H.; Leyva, A. Tetrahedron 2004, 60, 8553.
[37] Zhao, D.; Fei, Z.; Geldbach, T. J.; Scopelliti, R.; Dyson, P. J. J. Am. Chem. Soc. 2004, 126, 15876.
[38] Wang, R.; Piekarski, M. M.; Shreeve, J. M. Org. Biomol. Chem. 2006, 4, 1878.
[39] Wang, R.; Xiao, J.-C.; Twamley, B.; Shreeve, J. M. Org. Biomol. Chem. 2007, 5, 671.
[40] Zhou, L.; Wang, L. Synthesis 2006, 2653.
[41] Li, H.; Wang, L. Eur. J. Org. Chem. 2006, 5099.
[42] Lombardo, M.; Chiarucci, M.; Trombini, C. Green Chem. 2009, 11, 574.
[43] Wang, L.; Li, H.; Li, P. Tetrahedron 2009, 65, 364.
[44] Zhou, C.; Wang, J.; Li, L.; Wang, R.; Hong, M. Green Chem. 2011, 13, 2100.
[45] Li, P.; Wang, L. Adv. Synth. Catal. 2006, 348, 681.
[46] Zhang, L.; Wang, L.; Li, H.; Li, P. Synth. Commun. 2008, 38, 1498.
[47] Zhang, L.; Li, P.; Wang, L. Lett. Org. Chem. 2006, 3, 282.
[48] Miao, T.; Wang, L. Synthesis 2008, 363.
[49] Miao, T.; Wang, L. Tetrahedron Lett. 2007, 48, 95.
[50] Wang, Z.; Wang, L.; Li, P. Synthesis 2008, 1367.
[51] Wang, Z.; Wang, L.; Yan, J. Chin. J. Chem. 2008, 26, 1721.
[52] (a) Wang, M.; Li, P.; Wang, L. Eur. J. Org. Chem. 2008, 2255. (b) Li, P.; Wang, L. Tetrahedron 2007, 63, 5455.
[53] Li, P.; Wang, L.; Zhang, Y. Tetrahedron 2008, 64, 10825.
[54] Chen, W.; Li, P.; Wang, L. Tetrahedron 2011, 67, 318.
[55] Cai, M.; Sha, J.; Xu, Q. Tetrahedron 2007, 63, 4642.
[56] Zhao, H.; Yin, L.; Cai, M. Eur. J. Org. Chem. 2013, 1337.
[57] (a) Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J. M. Chem. Rev. 2011, 111, 3036. (b) Polshettiwar, V.; Varma, R. S. Green Chem. 2010, 12, 743. (c) Shylesh, S.; Schunemann, V.; Thiel, W. R. Angew. Chem., Int. Ed. 2010, 49, 3428. (d) Lu, A. H.; Salabas, E. L.; Schuth, F. Angew. Chem., Int. Ed. 2007, 46, 1222.
[58] Zheng, Y.; Stevens, P. D.; Gao, Y. J. Org. Chem. 2006, 71, 537.
[59] Baruwati, B.; Guin, D.; Manorama, S. V. Org. Lett. 2007, 9, 5377.
[60] Jin, M. J.; Lee, D. H. Angew. Chem., Int. Ed. 2010, 49, 1119.
[61] Li, P.; Wang, L.; Zhang, L.; Wang, G. Adv. Synth. Catal. 2012, 354 , 1307.
[62] Zhang, L.; Li, P.; Li, H.; Wang, L. Catal. Sci. Technol. 2012, 2, 1859.
[63] Zhang, Q.; Su, H.; Luo, J.; Wei, Y. Catal. Sci. Technol. 2013, 3, 235.
[64] Zolfigol, M. A.; Khakyzadeh, V.; Moosavi-Zare, A. R.; Rostami, A.; Zare, A.; Iranpoor, N.; Beyzavie, M. H.; Luque, R. Green Chem. 2013, 15, 2132.
[65] Esmaeilpoura, M.; Sardarian, A. R.; Javidi, J. J. Organomet. Chem. 2014, 749, 233.
[66] Zhang, L.; Li, P.; Liu, C.; Yang, J.; Wang, M.; Wang, L. Catal. Sci. Technol. 2014, 4, 1979.
[67] (a) Zhou, L.; Wang, L. Chem. Lett. 2007, 36, 628. (b) Mata, Y.; Pàmies, O.; Diéguez, M. Chem. Eur.-J. 2007, 13, 3296. (c) Miao, T.; Wang, L. Tetrahedron Lett. 2008, 49, 2173. (d) Mata, Y.; Diéguez, M.; Pàmies, O.; Claver, C. Org. Lett. 2005, 7, 5597.
[68] (a) List, B.; Lerner, R. A.; Barbas, III, C. F. J. Am. Chem. Soc. 2000, 122, 2395. (b) Li, P.; Wang, L.; Wang, M.; Zhang, Y. Eur. J. Org. Chem. 2008, 1157. (c) Cortez, G. S.; Tennyson, R. L.; Romo, D. J. Am. Chem. Soc. 2001, 123, 7945. (d) Li, P.; Wang, L.; Zhang, Y.; Wang, G.-W. Tetrahedron 2008, 64, 7633.
/
| 〈 |
|
〉 |