

吡喃盐化合物的合成及应用研究进展
收稿日期: 2014-09-10
修回日期: 2014-10-11
网络出版日期: 2014-10-21
基金资助
国家自然科学基金(Nos. 51003009, 20772014)、中央高校基本科研业务费(No. DUT14LK32)和辽宁省教育厅科学技术研究(No. L2014033)资助项目.
Research Progress on the Synthesis and Application of Pyrylium Salts
Received date: 2014-09-10
Revised date: 2014-10-11
Online published: 2014-10-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51003009, 20772014), the Fundamental Research Funds for the Central Universities of China (No. DUT14LK32) and the Science and Technology Research Foundation of Education Department of Liaoning Province (No. L2014033).
叶俊伟 , 王潇潇 , 高远 , 杨立健 , 林源 , 宁桂玲 . 吡喃盐化合物的合成及应用研究进展[J]. 有机化学, 2015 , 35(2) : 373 -383 . DOI: 10.6023/cjoc201409023
Pyrylium salts are a type of heterocyclic compounds with unique chemical reactivity and photophysical properties. They show great potential applications in the many fields such as organic synthesis, biosensor and organic optoelectronic materials. Based on our recent research results, in this review, the developments in the synthesis of both α-non-active pyrylium salts and α-active pyrylium salts are summarized, and the applications of some typical pyrylium salts in the organic synthesis, photosensitizers, ion liquid and fluorescent sensor are exemplified.
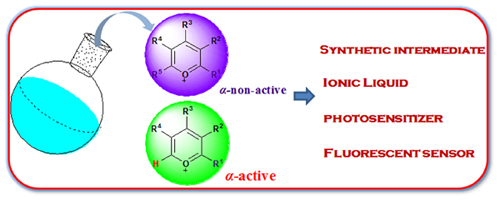
Key words: pyrylium salts; synthesis; α-active; α-non-active
[1] Binnemans, K. Chem. Rev. 2005, 105, 4148.
[2] Saeva, F. D.; Reynolds, G. A.; Kaszczuk, L. J. Am. Chem. Soc. 1982, 104, 3524.
[3] Pernak, J.; Swierczynska, A.; Kot, M.; Walkiewicz, F.; Maciejewski, H. Tetrahedron Lett, 2011, 52, 4342.
[4] Wang, Y.; He. Q. P.; Li, M. L.; Xu, X. J.; Yin, H. L. Chin. J. Org. Chem. 2008, 28, 718 (in Chinese). (王勇, 赫庆鹏, 李满林, 许小晶, 尹汉东, 有机化学, 2008, 28, 718.)
[5] Dimroth, K.; Greif, N.; Stade, W.; Steuber, F. W. Angew. Chem., Int. Ed. 1967, 6, 711.
[6] Abalos, T.; Jimenez, D.; Martinez-Manez, R.; Ros-Lis, J. V.; Royo, S.; Sancenon, F.; Soto, J.;Costero, A. M.; Gil, S.; Parra, M. Tetrahedron Lett. 2009, 50, 3885.
[7] Zhang, J. L.; Zhu, Z. G. Dyes Pigm. 1995, 27, 263.
[8] Gavrilyuk, S.; Polyutov, S.; Jha, P. C.; Rinkevicius, Z.; Agren, H.; Gei'mukhanov, F. J. Phys. Chem. A 2007, 111, 11961.
[9] Jayanthi, S. S.; Ramamurthy, P. J. Phys. Chem. A 1998, 102, 511.
[10] Song, X. N.; Mo, L.; Hu, Z. L.; Yang, Z. Y.; Yao, Z. J. Acta Chim. Sinica 2011, 69, 680 (in Chinese). (宋晓男, 莫磊, 胡治隆, 杨震宇, 姚祝军, 化学学报, 2011, 69, 680.)
[11] Li, F. Y.; Zheng, J.; Huang, C. H. J. Phys. Chem. B 2000, 104, 5090.
[12] Scaramuzzo, F. A.; Gonzalez-Campo, A.; Wu, C. C.; Velders, A. H.; Subramaniam, V.; Doddi, G.; Mencarelli, P.; Barteri, M.; Jonkheijm, P.; Huskens, J. Chem. Commun. 2010, 46, 4193.
[13] Zhou, T. L.; Jia, T.; Zhao, S. S.; Guo, J. H.; Zhang, H. Y.; Wang, Y. Cryst. Growth Des. 2012, 12, 179.
[14] Müller, C.; Freixa, Z.; Lutz, M.; Spek, A. L.; Vogt, D.; van Leeuwen, P. W. N. M. Organometallics 2008, 27, 834.
[15] Liebscher, J.; Harumann, H. Z. Chem. 1973, 13, 342.
[16] Balaban, T. S.; Balaban, A. T. Sci. Synth. 2003, 14, 11.
[17] Ning, G. L.; Li, X. C.; Munakata, M.; Gong, W. T.; Maekawa, M.; Kamikawa, T. J. Org. Chem. 2004, 69, 1432.
[18] Ye, J. W.; Zhang, X. D.; Deng, D.; Ning, G. l.; Liu, T. Q.; Zhuang, M. L.; Yang, L. J.; Gong, W. T.; Lin, Y. RSC Adv. 2013, 3, 8232.
[19] Gong, W. T.; Ning, G. L.; Li, X. C.; Wang, L.; Lin, Y. J. Org. Chem. 2005, 70, 5768.
[20] Yang, L. J.; Ye, J. W.; Gao, Y.; Deng,D.; Gong, W. T.; Li, Y.; Ning, G. L. Tetrahedron Lett. 2013, 54, 2967.
[21] Xu, T.; Gong, W. T.; Ye, J. W.; Li, Y.; Ning, G. L. Organometallics 2010, 29, 6744.
[22] Li, G.; Gong, W. T.; Ye, J. W.; Li, Y.; Ning, G. L. Synth. Commun. 2012, 42, 480.
[23] Zhang, X. D.; Ye, J. W.; Wang, S. N.; Gong, W. T.; Li, Y.; Ning, G. L. Org. Lett. 2011, 13, 3608.
[24] Yang, L. J.; Ye, J. W.; Gao, Y.; Deng,D.; Li, Y.; Ning, G. L. Eur. J. Org. Chem. 2014, 3, 515.
[25] Li, G.; Gong, W. T.; Ye, J. W.; Lin, Y.; Ning, G. L. Chin. J. Org. Chem. 2011, 31, 216 (in Chinese). (李刚, 贡卫涛, 叶俊伟, 林源, 宁桂玲, 有机化学, 2011, 31, 216.)
[26] Krivun, S. V.; Buryak, A. I.; Baranov, S. N. Chem. Heterocycl. Compd. 1973, 9, 1191.
[27] Balaban, A. T.; Dinculescu, A.; Dorofeenko, G. N.; Fischer, G. W.; Koblik, A. V.; Mezheritskii, V. V.; Schroth, W. Adv. Heterocycl. Chem. 1982, (Suppl. 2), 184.
[28] Vanallan, J. A.; Reynolds, G. A. J. Org. Chem. 1968, 33, 1102.
[29] Fakis, M.; Polyzos, J; Tsigaridas, G.; Parthenios, J.; Fragos, A.; Giannetas, V.; Persephonis, P.; Mikroyannidis, J. Chem. Phys. Lett. 2000, 323, 111.
[30] Cheng, X. H.; Hoger, S.; Fenske, D. Org. Lett. 2003, 5, 2587.
[31] Krivun S. V.; Shiyan, Z. V.; Dorofeenko, G. N.. Zh. Obshch. Khim. 1964, 34, 167.
[32] Balaban, A. T.; Nenitzesco, C. D. J. Chem. Soc. 1961, 3553.
[33] Balaban, A. T.; Nenitzescu, C. D. Org. Synth. 1964, 44, 98.
[34] Hopf, P. P.; Le Fevre, R. J. W. J. Chem. Soc. 1938, 1989.
[35] Katritzky, A. R.; Vassilatos, S. N.; Alajarin, C. M. Org. Magn. Res. 1983, 21, 587.
[36] van der Velde, N. A.; Korbitz, H. T.; Garner, C. M. Tetrahedron Lett. 2012, 53, 5742.
[37] Tovar, J. D.; Swager, T. M. J. Org. Chem. 1999, 64, 6499.
[38] Shaw, M.J.; Mertz, J. Organometallics 2002, 21, 3434.
[39] Lombard, R. Stephan, J. P. Bull. Soc. Chim. Fr. 1958,1458.
[40] Kuhnert, N.; Clifford, M. N.; Radenac, A.G. Tetrahedron Lett. 2001, 42, 9261.
[41] Bello, A. M.; Kotra, L. P. Tetrahedron Lett. 2003, 44, 9271.
[42] Prasad, N.; Main, L.; Nicholson, B. K.; McAdam, C. J. J. Organomet. Chem. 2010, 695, 1961.
[43] Liebscher, J.; Hartmann, H. Z. Chem. 1973, 13, 132.
[44] Schroth, W.; Fischer, G. W.; Rottmann, J. Chem. Ber. 1969, 102, 1201.
[45] Dorofeenko, G. N.; Mezheritskii, V. V. Khim. Geterotsikl. Soedin. 1970, 2, 217
[46] Simalty, M.; Carretto, J.; Sib, S. Bull. Soc. Chim. Fr. 1970, 11, 3920.
[47] Laine, P.; Bedioui, F.; Ochsenbein, P.; Marvaud, V.; Bonin, M.; Amouyal, E. J. Am. Chem. Soc. 2002, 124, 1364.
[48] Bhowmik, P. K.; Han, H.; Cebe, J. J.; Nedeltchev, I. K.; Kang, S. W.; Kumar, S. Macromolecules 2004, 37, 2688.
[49] Wu, D. Q.; Zhi, L. J.; Bodwell, G. J.; Cui, G. L.; Tsao, N.; Müller, K. Angew. Chem., Int. Ed. 2007, 46, 5417.
[50] Uncuta, C.; Caproiu, M. T.; Campeanu, V.; Petride, A.; Danila, M. G.; Plaveti, M.; Balaban, A. T. Tetrahedron 1998, 54, 9747.
[51] Mouradzadegun, A.; Dianat, S. J. Heterocycl. Chem. 2009, 46, 778.
[52] Tolmachev, A. I.; Koslov, E. S. Zh. Obshch. Khim. 1967, 37, 1922.
[53] Markl, G.; Lieb, F.; Merz, A. Angew. Chem., Int. Ed. 1967, 6, 458.
[54] Krivun, S. V.; Voziyano, O. F.; Baranov, S. N. Zh. Obshch. Khim. 1972, 42, 58.
[55] Kamata, M.; Kaneko, J.; Hagiwara, J.; Akaba, R. Tetrahedron Lett. 2004, 45, 7423.
[56] Miranda, M. A.; Garcia, H. Chem. Rev. 1994, 94, 1063.
[57] Branchi, B.; Bietti, M.; Ercolani, G.; Izquierdo, M. A.; Miranda, M. A.; Stella, L. J. Org. Chem. 2004, 69, 8874.
[58] Liang, S.; Zhu, J. K.; Hurst, J. K. Langmuir. 2012, 28, 12171.
[59] Zlotin, S. G.; Makhova, N. N. Mendeleev Commun. 2010, 20, 63.
[60] Saeva, F. D.; Reynolds, G. A.; Kaszczuk, L. J. Am. Chem. Soc. 1982, 104, 3524.
[61] Gionis, V.;Fugnitto, R.; Strzelecka, H.; Le Barny, P. Mol. Cryst. Liq. Cryst. 1983, 95, 351.
[62] Veber, M.; Berruyer, G. Liq. Cryst. 2000, 27, 671.
[63] Pernak, J.; Swierczynska, A.; Kot, M.; Walkiewicz, F.; Maciejewski, H. Tetrahedron Lett. 2011, 52, 4342.
[64] Chen, Y.; Wu, S. K. Chem. J. Chin. Univ. 1996, 17, 1622 (in Chinese). (陈懿, 吴世康, 高等学校化学学报, 1996, 17, 1622.)
[65] Haucke, G.; Czerney, P.; Cebulla, F. Ber. Bunsen-Ges. Phys.Chem. 1992, 96, 880.
[66] Azab, H. A.; El-Korashy, S. A.; Anwar, Z. M.; Khairy, G. M.; Duerkop, A. J. Photochem. Photobiol., A 2012, 243, 41.
[67] Sheng, Y. H.; Ren, Y. J. Phys. Chem. A 2012, 116, 5420.
[68] Prust, E. E.; Carlson, E. J.; Dahl, B. J. Tetrahedron Lett. 2012, 53, 6433.
/
| 〈 |
|
〉 |