

N-烃基-5-芳基吲哚-3-甲醛的合成和光谱性质
收稿日期: 2014-07-09
修回日期: 2014-09-28
网络出版日期: 2014-10-22
基金资助
广东省大学生创新(No. 1055912003)资助项目.
Syntheses and Spectral Properties of N-Alkyl-5-arylindole-3-carboxaldehydes
Received date: 2014-07-09
Revised date: 2014-09-28
Online published: 2014-10-22
Supported by
Project supported by the University Student Innovative Program of Guangdong Province (No. 1055912003).
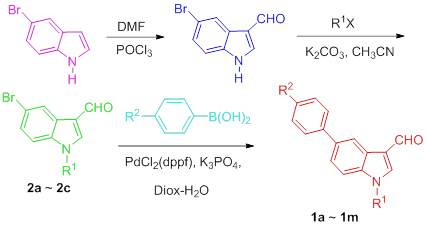
以5-溴吲哚为原料, 经甲酰化、烷基化、PdCl2(dppf)催化下的Suzuki偶联反应, 合成了系列N-烃基-5-芳基吲哚-3-甲醛. 考察了催化剂、碱试剂和溶剂的选择与用量, 以及反应物配比等因素对Suzuki偶联反应的影响. 测定了标题化合物在乙腈中的紫外-可见吸收光谱和荧光发射光谱.
关键词: N-烃基-5-芳基吲哚-3-甲醛; 光谱性质; Suzuki偶联反应; 合成
林志强 , 曾向潮 , 蒙玉霞 . N-烃基-5-芳基吲哚-3-甲醛的合成和光谱性质[J]. 有机化学, 2015 , 35(2) : 490 -496 . DOI: 10.6023/cjoc201407011
Using 5-bromoindole as raw material and PdCl2(dppf) as catalyst, N-alkyl-5-arylindole-3-carboxaldehydes were synthesized by the formylation, alkylation and Suzuki coupling reaction. The effects of catalysts, alkali reagents, solvents and the molar ratio of reactants on Suzuki coupling reaction have been surveyed. UV-Vis and fluorescence spectra of the title compounds in acetonitrile were also investigated.

[1] Tiwari, R. K.; Singh, D.; Singh, J.; Yadav, V.; Pathak, A. K.; Dabur, R.; Chhillar, A. K.; Singh, R.; Sharma, G. L.; Chandra, R.; Verma, A. K. Bioorg. Med. Chem. Lett. 2006, 16, 413.
[2] Zhai, X.; Fang, Y.-Y.; Leng, X.; Ma, C.-L.; Zeng, L.-L.; Gong, P. Chin. J. Med. Chem. 2008, (4), 254 (in Chinese). (翟鑫, 房元英, 冷雪, 马崇雷, 曾兰兰, 宫平, 中国药物化学杂志, 2008, (4), 254.)
[3] Tiwari, R. K.; Verma, A. K. Bioorg. Med. Chem. Lett. 2006, 14, 2747.
[4] Chen, L.-R.; Wang, Y.-C.; Lin, Y.-W.; Chou, S.-Y.; Chen, S.-F.; Liu, L.-T.; Wu, Y.-T.; Kuo, C.-J.; Chen, T.-S.; Juang, S.-H. Bioorg. Med. Chem. Lett. 2005, 15, 3058.
[5] Giampieri, M.; Balbi, A.; Mazzei, M.; Colla, P. L.; Ibba, C.; Loddo, R. Antiviral Res. 2009, 83, 179.
[6] Dahlhaus, J.; Georgi, G.; Mueller, J. D. DE 10124600, 2002 [Chem. Abstr. 2002, 137, 386319].
[7] Staub, R. E.; Feng, C.; Onisko, B.; Bailey, G. S.; Firestone, G. L.; Bjeldanes, L. F. Chem. Res. Toxicol. 2002, 15, 101.
[8] Xu, M.-H.; Zhang, G.-Y.; Xie, Z.-X.; He, C.-M. Chin. J. Digestion 2002, 10, 6052 (in Chinese). (徐美华, 张桂英, 谢兆霞, 何春梅, 中华消化杂志, 2002, 10, 6052.)
[9] James, D. A.; Koya, K.; Li, H.; Liang, G.; Xia, Z.; Ying, W.; Wu, Y.; Sun, L. Bioorg. Med. Chem. Lett. 2008, 18, 1784.
[10] Duan, C.-F.; Yang, Y.-J. Acta Pharm. Sin. 1996, 31, 182 (in Chinese). (段传风, 杨依军, 药学学报, 1996, 31, 182.)
[11] Horiuchi, T.; Miura, H.; Uehida, S. Chem. Commun. 2003, 24, 3036.
[12] Horiuchi, T.; Miura, H.; Sumioka, K.; Uchida, S. J. Am. Chem. Soc. 2004, 126, 12218.
[13] Zhan, W.-S.; Pan, S.; Li, Y.-Z.; Chen, M.-D. Acta Phys.-Chim. Sin. 2009, 25, 2087 (in Chinese). (詹卫伸, 潘石, 李源作, 陈茂笃, 物理化学学报, 2009, 25, 2087.)
[14] Ge, Y.-H.; Wu, Y.-M.; Xue, Z.-J. Chin. J. Org. Chem. 2006, 26, 563 (in Chinese). (葛裕华, 吴亚明, 薛忠俊, 有机化学, 2006, 26, 563.)
[15] Da, B.-L. Flavour Fragrance Cosmetics 1995, 28, 22 (in Chinese). (笪宝林, 香料香精化妆品, 1995, 28, 22.)
[16] Zhang, R.; Li, J.-T.; Fu, C.-L.; Luo, X.-T. Mater. Rev. 2011, 25, 61 (in Chinese). (张蓉, 李锦堂, 傅翠梨, 罗学涛, 材料导报, 2011, 25, 61.)
[17] Yu, K.; Guan, S.-X.; Zhang, H.-W.; Zhou, B.-B.; Li, L. Nat. Sci. J. Harbin Normal Univ. 2006, 22, 70 (in Chinese). (于凯, 关淑霞, 张宏伟, 周百斌, 李玲, 哈尔滨师范大学自然科学学报, 2006, 22, 70.)
[18] Kiliç, Z.; Isgör, Y. G.; Ölgen, S. Arch. Pharm. 2009, 342, 333.
[19] Go, M. L.; Leow, J. L.; Gorla, S. K.; Schüller, A. P.; Wang, M.; Casey, P. J. J. Med. Chem. 2010, 53, 6838.
[20] Winter-Vann, A. M.; Baron, R. A.; Wong, W.; Dela C. J.; York, J. D.; Gooden, D. M.; Bergo, M. O.; Young, S. G.; Toone, E. J.; Casey, P. J. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 4336.
[21] Valdenaire, A.; Pothier, J.; Renneberg, D.; Riederer, M. A.; Peter, O.; Leroy, X.; Gnerre, C.; Fretz, H. Bioorg. Med. Chem. Lett. 2013, 23, 944.
[22] Li, Q. Q.; Zou, J. H.; Chen, J. W.; Liu, Z. J.; Qin, J. G.; Li, Z.; Cao, Y. Phys. Chem. B 2009, 113, 5816.
[23] Lo, K. K.-W.; Chung, C.-K.; Zhu, N.-Y. Chem. Eur. J. 2003, 9, 475.
[24] Chen, F.-F.; Bian, Z.-Q.; Liu, Z.-W.; Nie, D.-B. Chen, Z.-Q.; Huang, C.-H. Inorg. Chem. 2008, 47, 2507.
[25] Martin, A. R.; Yang, Y. Acta Chem. Scand. 1993, 47, 221.
[26] Wallow, T. I.; Novak, B. M. J. Org. Chem. 1994, 59, 5034.
[27] Gronowitz, S.; Hörnfeldt, A. B.; Yang, Y. H. Chem. Scr. 1988, 28, 281.
[28] Zhang, H.; Kwong, F. Y.; Tian Y.; Chan, K. S. J. Org. Chem. 1998, 63, 6886.
[29] Gao, F.-Q.; He, H.-J.; Wang, X.-M.; Guo, Q. Fine Chem. 2012, 29, 1142 (in Chinese). (高丰琴, 何汉江, 王小明, 郭强, 精细化工, 2012, 29, 1142.)
[30] Xin, B.-W. Chin. J. Appl. Chem. 2008, 25, 895 (in Chinese). (辛炳炜, 应用化学, 2008, 25, 895.)
[31] DeVasher, R. B.; Moore, L. R.; Shaughnessy, K. H. J. Org. Chem. 2004, 69, 7919.
[32] Bumagin, N. A.; Korolev, D. N. Tetrahedron Lett. 1999, 40, 3057.
[33] Mao, P.; Yang, L.-R.; Liu, X.-J.; Cai, Y.-J.; Zhao, C.-L.; Song, M.-P. Chin. J. Org. Chem. 2011, 31, 1828 (in Chinese). (毛璞, 杨亮茹, 刘秀君, 蔡雅静, 赵成磊, 宋毛平, 有机化学, 2011, 31, 1828.)
[34] Haruaki, I; Kazuaki, Y; Jun'ichi, K. Tetrahetron 2012, 68, 6186.
[35] Zhang, W.; Jin, X.-L.; Yu, X.-G.; Zhou, J.; Tang, G.-P.; Peng, D.-H.; Hu, J.-M.; Zhong, C.-F. J. Organomet. Chem. 2014, 749, 26.
/
| 〈 |
|
〉 |