

季膦离子液体促进的Morita-Baylis-Hillman反应
收稿日期: 2014-10-14
修回日期: 2014-11-16
网络出版日期: 2014-12-08
基金资助
山西省重点学科基金(No. 20141010)、研究生创新项目(No. 020352901016)、忻州师范学院重点学科基金(No. XK201304)资助项目.
Phosphonium Ionic Liquids-Accelerated Morita-Baylis-Hillman Reaction
Received date: 2014-10-14
Revised date: 2014-11-16
Online published: 2014-12-08
Supported by
Project supported by the Key Discipline Project of Shanxi Province Education Department (No. 20141010), the Graduates Innovation Project of Shanxi Province (No. 020352901016) and the Key Discipline Project of Xinzhou Teachers University (No. XK201304).
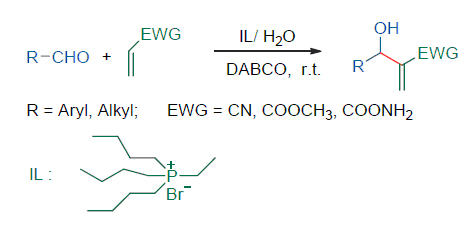
膦类离子液体, 因其好的热稳定性以及在弱碱性介质中的惰性等优异特点, 正在受到越来越多的关注. 以廉价的三丁基膦和各种卤代烃为原料, 制备了一系列季膦离子液体, 然后以其与水组成的复合体系为反应介质, 在催化剂1,4-二氮杂二环[2.2.2]辛烷(DABCO)作用下, 进行了各种醛与丙烯腈、丙烯酸甲酯及丙烯酰胺的Morita-Baylis-Hillman 反应研究. 实验结果表明, 在室温条件下, 溴化乙基三丁基膦与水组成的复合溶剂体系, 有效地促进了各种芳香醛及脂肪醛的Morita-Baylis-Hillman 反应, 不仅反应速度快, 而且目标产物产率高.
关键词: 膦类离子液体; 水; 醛; Morita-Baylis-Hillman反应
赵三虎 , 王豆 , 王敏 , 康锦 , 张立伟 . 季膦离子液体促进的Morita-Baylis-Hillman反应[J]. 有机化学, 2015 , 35(4) : 865 -874 . DOI: 10.6023/cjoc201410020
Phosphonium ionic liquids, due to their superior thermal stability and an inertness in weak basic reaction media, are receiving more and more attention. A series of phosphonium ionic liquids have been prepared starting from cheap tributylphosphine with various halogenated hydrocarbon. Then using 1,4-diazabicyclo[2.2.2]octane (DABCO) as catalyst and water-phosphonium ionic liquid composite system as reaction media, Morita-Baylis-Hillman reactions of a variety of aldehydes with acrylonitrile, methyl acrylate or acrylamide were studied. Experimental results showed that the composite system of phosphonium ionic liquid with water can effectivily accelerate the Morita-Baylis-Hillman reactions of aromatic aldehydes and aliphatic aldehydes, and fast reaction rate and high yield were obtained.

[1] Huddleston, J. G.; Visser, A. E.; Reichert, W. M.; Willauer, H. D.; Br-oker, G. A.; Rogers, R. D. Green Chem. 2001, 3, 156.
[2] Wilkes, J. S.; Zaworotko, M. J. J. Chem. Soc., Chem. Commun. 1992, 13, 965.
[3] Welton, T. Chem. Rev. 1999, 99, 2071.
[4] (a) Freudenmann, D.; Wolf, S.; Wolff, M.; Feldmann, C. Angew. Chem., Int. Ed. 2011, 47, 11050.
(b) Gunaratne, H. Q. N.; Langrick, C. R.; Puga, A. V.; Seddon, K. R.; Whiston, K. Green Chem. 2013, 15, 1166.
(c) Zhang, C.; Shi, R.; Chen, C.; Jin, C. Chin. J. Org. Chem. 2013, 33, 611 (in Chinese).
(张传越, 石若冰, 陈才元, 金传明, 有机化学, 2013, 33, 611.)
(d) Sheng, W.; Tian, F.; Du, Y.; Wu, C. Chin. J. Org. Chem. 2013, 33, 835 (in Chinese).
(盛万里, 田福利, 杜玉英, 吴春雨, 有机化学, 2013, 33, 835.)
(e) Song, Y.; Wang, X.; Huang, C.; Liang, F.; Yu, Z.; Chen, B. Chin. J. Org. Chem. 2013, 33, 1715 (in Chinese).
(宋彦磊, 王新承, 黄崇品, 梁凤兵, 毓志超, 陈标华, 有机化学, 2013, 33, 1715.)
(f) Zhao, D.; Wu, Y.; Chen, T.; Dai, L.; Wang, Y. Chin. J. Org. Chem. 2013, 33(08), 1791 (in Chinese).
(赵东旺, 吴悦彤, 陈婷婷, 戴立益, 王媛媛, 有机化学, 2013, 33(08), 1791.)
(g) Song, C.; Liu, H.; Li, Y.; Ge, S.; Wang, H.; Zhu, W.; Chang, Y.; Han, C.; Li, H. Chin. J. Chem. 2013, 32(5), 434.
[5] (a) Rosen, B. A.; Salehi-Khojin, A.; Thorson, M. R.; Zhu, W.; Whipple, D. T.; Kenis, P. J. A.; Masel, R. Science 2011, 334, 643.
(b) Gaucho, V.; Schmitzer, A. R. J. Org. Chem. 2012, 11, 4917.
(c) Hallett, J. P.; Welton, T. Chem. Rev. 2011, 111, 3508.
[6] (a) Miao, Q.; Zhang, S.; Xu, H.; Zhang, P.; Li, H. Chem. Commun. 2013, 49, 6980.
(b) Wu, W.; Zhang, X.; Hu, Y.; Jin, B.; Hua, J. Chin. J. Chem. 2013, 31(3), 388.
[7] (a) Liu, Y.-L.; Tseng, M.-C.; Chu, Y.-H. Chem. Commun. 2013, 49, 2560.
(b) Anderson, J. L.; Armstrong, D. W.; Wei, G.-T. Anal. Chem. 2006, 78, 2892.
(c) Kuang, M.; Zhang, Y.; Yang, P.; Lu, H. Acta Chim. Sinica 2013, 71, 1007 (in Chinese).
(匡敏, 张莹, 杨芃原, 陆豪杰, 化学学报, 2013, 71, 1007.)
(d) Gao, X.; Fan, J.; Wang, X.; Zhang, Y. Acta Chim. Sinica 2013, 71, 1411 (in Chinese).
(高霞, 樊静, 王小龙, 张艳树, 化学学报, 2013, 71, 1411.)
(e) Li, Y.; Qi, Li.; Shen, Y.; Zhang, H.; Ma, H. Chin. J. Chem. 2014, 32, 619 (in Chinese).
[8] (a) Drewes, S. E.; Roos, G. H. P. Tetrahedron 1988, 44, 4653.
(b) Basavaiah, D.; Rao, P. D.; Hyma, R. S. Tetrahedron 1996, 52, 8001.
(c) Zhang, A. M.; Wang, W.; Lin, G. Q. Chin. J. Org. Chem. 2001, 21, 134 (in Chinese).
(张爱民, 王伟, 林国强, 有机化学, 2001, 21, 134.)
(d) Cai, J. X.; Zhou, Z. H.; Tang, C. C. Chem. Res. 2001, 12(2), 54 (in Chinese).
(蔡觉晓, 周正洪, 唐除痴, 化学研究, 2001, 12(2), 54.)
(e) Basavaiah, D.; Rao, A. J.; Satyanarayana, T. Chem. Rev. 2003, 103, 811.
(f) Zhao, S. H.; Zhao, M. G.; Zhang, H. R.; Chen, Z. B. Chin. J. Org. Chem. 2007, 27, 322 (in Chinese).
(赵三虎, 赵明根, 张海容, 陈兆斌, 有机化学, 2007, 27, 322.)
[9] (a) Aggarwal, V. K; Emme, I.; Fulford, S. Y. J. Org. Chem. 2003, 68, 692.
(b) Mi, X.; Luo, S.; Xu, H.; Zhang, L.; Cheng, J.-P. Tetrahedron 2006, 62, 2537.
(c) Ma, G.-N.; Jiang, J.-J.; Shi, M. Chem. Commun. 2009, 45, 5496.
(d) Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447.
(e) Basavaiah, D.; Veeraraghavaiah, G. Chem. Soc. Rev. 2012, 41, 68.
[10] Rosa, J. N.; Afonso, C. A. M.; Santos, A. G. Tetrahedron 2001, 57, 4189.
[11] Aggarwal, V. K.; Emme, I.; Mereu, A. Chem. Commun. 2002, 1612.
[12] Hsu, J.; Yen, Y.; Chu, Y. Tetrahedron Lett. 2004, 45, 4673.
[13] Jur?ík, V.; Wilhelm, R. Green Chem. 2005, 7, 844.
[14] (a) Zhao, S.; Bie, H.; Chen, Z. Org. Prep. Proced. Int. 2005, 37, 231.
(b) Zhao, S.; Zhang, H.; Feng, L.; Chen, Z. J. Mol. Catal. A: Chem. 2006, 258, 251.
[15] (a) Zhao, S.; Xu, X.; Zheng, L.; Liu, H. Ultrason. Sonochem. 2010, 17, 685.
(b) Zhao, S.; Wang, X.; Zhang, L. RSC Adv. 2013, 3, 11691.
[16] Bellina, F.; Chiappe, C.; Lessi, M. Green Chem. 2012, 14, 148.
[17] Byun, H. S.; Reddy, K. C.; Bittman, R. Tetrahedron Lett. 1994, 35, 1371.
[18] (a) Yu, C. Z.; Liu, B.; Hu, L. Q. J. Org. Chem. 2001, 66, 5413.
(b) Luo, S.; Zhang, B.; Cheng, J.-P. Tetrahedron Lett. 2002, 43, 7369.
(c) Cai, J.; Zhou, Z.; Zhao, G.; Tang, C. Org. Lett. 2002, 4, 4723.
(d) Luo, S.; Wang, P. G.; Cheng, J.-P. J. Org. Chem. 2004, 69, 555.
(e) Yi, F. H.; Zhang, X.; Sun, H.; Chen, S. Acta Chim. Sinica 2012, 70, 741 (in Chinese).
(易封萍, 张旋, 孙海洋, 陈世洪, 化学学报, 2012, 70, 741.)
[19] Zhao, S.; Zhang, Q.; Duan, X.; Feng, L. Synth. Commun. 2011, 41, 3289.
[20] Johnson, C. L.; Donkor, R. E.; Nawaz, W.; Karodia, N. Tetrahedron Lett. 2004, 45, 7359.
[21] Bradaric, C. J.; Downard, A.; Kennedy, C.; Robertson, A. J.; Zhou, Y. Green Chem. 2003, 5, 143
[22] Loris, A.; Perosa, A.; Selva, M.; Tundo, P. J. Org. Chem. 2004, 69, 3953.
[23] Sesto, R. E. D.; Corley, C.; Robertson, A.; Wilkes, J. S. J. Organomet. Chem. 2005, 690, 2536.
[24] de Souza, R. O. M. A.; Meireles, B. A.; Aguiar, L. C. S.; Vasconcellos, M. L. A. A. Synthesis 2004, 1596.
[25] Yang, N.; Gong, H.; Tang, W.; Fan, Q.; Cai, C.; Yang, L. J. Mol. Catal. A: Chem. 2005, 233, 55.
/
| 〈 |
|
〉 |