

石蒜碱的全合成及结构修饰研究进展
收稿日期: 2014-09-24
修回日期: 2014-10-29
网络出版日期: 2014-12-10
基金资助
哈尔滨商业大学博士科研启动项目(No.14LG12)、哈尔滨商业大学青年教师自然科学基金(No.HCUL2013019)资助项目.
Progresses in the Total Synthesis and Structure Modification Studies of Lycorine
Received date: 2014-09-24
Revised date: 2014-10-29
Online published: 2014-12-10
Supported by
Project supported by the Harbin University of Commerce Doctoral Research Project (No.14LG12) and the Natural Science Fund for Young Teachers of Harbin University of Commerce (No.HCUL2013019).
刘颖杰 , 丁泽洋 , 谭冲 , 袁洪亮 , 季宇彬 . 石蒜碱的全合成及结构修饰研究进展[J]. 有机化学, 2015 , 35(5) : 1009 -1021 . DOI: 10.6023/cjoc201409038
Lycorine is a very important class of isoquinoline alkaloids of the amaryllidaceae alkaloids. In recent years, pharmaceutical and organic chemists discovered that lycorine and their derivatives have anti-tumor, anti-inflammatory, inhibiting acetylcholinesterase, anti-malarial, cardiovascular protection and other effects. Because of its complex and unique molecular structure and extensive pharmaceutical activities, lycorine has attracted considerable research effort. The recent progresses in the synthesis and structural modification of lycorine are summarized in this review.
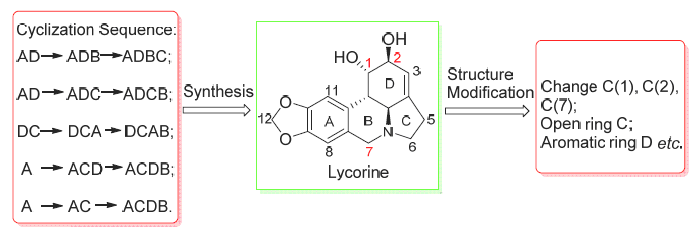
Key words: lycorine; total synthesis; derivatives; structure modification
[1] Huang, B. X.; Fu, S. G.; Zhu, P. L. Jiangxi Forestry Sci. Technol. 2012, 40(1), 22 (in Chinese).
(黄宝祥, 符树根, 朱培林, 江西林业科技, 2012, 40(1), 22.)
[2] Cook, J. W.; Loudon, J. D. In The Alkaloids, Eds.: Manske, R. H. F.; Holmes, H. L., Academic Press, New York, 1952, Vol. II, pp. 331~352.
[3] Kondo, H.; Uyeo, S. Chem. Ber. 1935, 68, 1756.
[4] Nakagawa, Y.; Uyeo, S. J. Chem. Soc. 1959, 3736.
[5] Lamoral-Theys, D.; Decaestecker, C.; Mathieu, V.; Dubois, J.; Kornienko, A.; Kiss, R.; Evidente, A.; Potter, L. Mini-Rev. Med. Chem. 2010, 10, 41.
[6] Cedron, J. C.; Gutierrez, D.; Flores. N.; Ravelo, A. G.; Estevez- Braun, A. Bioorg. Med. Chem. 2010, 18, 4694.
[7] Toriizuka, Y.; Kinoshita, E.; Kogure, N.; Kitajima, M.; Ishiyama, A.; Otoguro, K.; Yamada, H.; Omura, S.; Takayama, H. Bioorg. Med. Chem. 2008, 16, 10182.
[8] Citoglu, G.; Tanker, M.; Gumusel, B. Phytother. Res. 1998, 12, 205.
[9] Tanker, M.; Citoglu, G.; Gumuhel, B.; Hener, B. Int. J. Pharmacogn. 1996, 34, 194.
[10] Kretzing, S.; Abrahama, G.; Seiwert, B.; Ungemach, F. R.; Krugel, U.; Regenthal, R. Toxicon 2011, 57, 117.
[11] Hwang, Y. C.; Chu, P. L.; Yang, W.; Chen, W.; Yates, M. V. Antiviral Res. 2008, 77, 232.
[12] Li, S. Y.; Chen, C.; Zhang, H.; Guo, H.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.; Yu, J.; Xiao, P.; Li, R.; Tan, X. Antiviral Res. 2005, 67, 18.
[13] Renard-Nozaki, J.; Kim, T.; Imakura, Y.; Kihara, M.; Kobayashi, S. Res. Virol. 1989, 140, 115.
[14] Zhou, M.; Deng, L.; Kashanchi, F.; Brady, J. N.; Shatkin, A. J.; Kumar, A. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 12666.
[15] Gabrielsen, B.; Monath, T. P.; Huggins, J. W.; Kefauver, D. F. J. Nat. Prod. 1992, 55, 1569.
[16] Liu, J.; Li, Y.; Tang, L. J; Zhang, G. P.; Hu, W. X. Biomed. Pharmacother. 2007, 61, 229.
[17] Min, B. S.; Gao, J. J.; Nakamura, N.; Kim, Y. H.; Hattori, M. Chem. Pharm. Bull. 2001, 49, 1217.
[18] Cao, Z.; Yu, D.; Fu, S.; Zhang, G.; Pan, Y.; Bao, M,; Tu, J.; Shang, B.; Guo, P.; Yang, P.; Zhou, Q. Toxicol. Lett. 2013, 218, 174.
[19] Chapsal, B. D.; Ojima, I. Org. Lett. 2006, 8, 1395.
[20] Fujioka, H.; Murai, K.; Ohba, Y.; Hirose, H.; Kita, Y. Chem. Commun. 2006, 832;
[21] Dong, L.; Xu, Y. J.; Cun, L. F.; Cui, X.; Jiang, Y. Z.; Gong, L. Z. Org. Lett. 2005, 7, 4285;
[22] Banwell, M. G.; Harvey, J. E.; Hockless, D. C. R. J. Org. Chem. 2000, 65, 4241.
[23] Tsuda, Y.; Sano, T.; Taga, J.; Isobe, K.; Toda, J.; Irie, H.; Tanaka, H.; Takagi, S.; Yamaki, M.; Murata, M. J. Chem. Soc., Chem. Commun. 1975, 933.
[24] Trossell, K. Tetrahedron Lett. 1974, 623.
[25] Umezawa, B.; Hoshino, O.; Sawaki, S.; Sashida, H.; Mori, K.; Hamada, Y.; Kotera, K.; Iitaka, Y. Tetrahedron 1984, 40, 1783.
[26] Møller, O.; Steinberg, E. M.; Torssell, K. Acta Chem. Scand. Sect. B 1978, 32, 98.
[27] Yamada, K.; Yamashita, M.; Sumiyoshi, T.; Ni-Shimura, K.; Tomioka, K. Org. Lett. 2009, 11, 1631.
[28] Magnus, P.; Sebhat, I. K. J. Am. Chem. Soc. 1998, 120, 5341.
[29] Sun, Z. W.; Zhou, M. T.; Li, X.; Meng, X. L.; Peng, F. Z.; Zhang, H. B.; Shao, Z. H. Chem. Eur. J. 2014, 20, 6112.
[30] Schultz, A. G.; Holoboski, M. A.; Smyth, M. S. J. Am. Chem. Soc. 1996, 118, 6210.
[31] Pozsgay, V.; Ohgi, T.; Hecht, S. M. J. Org. Chem. 1981, 46, 3763.
[32] Boeckman, R. J.; Goldstein, S. W.; Walters, M. A. J. Am. Chem. Soc. 1988, 110, 8250.
[33] Elgorash, E. E.; Stafford, G. I.; Van Staden, J. Planta Med. 2004, 70(3), 260.
[34] Wang, P.; Li, L. F.; Wang, Q. Y.; Shang, L. Q.; Shi, P. Y.; Yin, Z. ChemMedChem 2014, 9, 1522.
[35] Tan, C. X.; Schrader, K. K.; Mizuno, C. S.; Rimando, A. M. J. Agric. Food Chem. 2011, 59, 5977.
[36] Evdokimov, N. M.; Theys, D. L.; Mathieu, V.; Andolfi, A.; Frolova, L. V.; Pelly, S. C.; Otterlo, W.; Magedov, I. V.; Kiss, R.; Evidente, A.; Kornienko, A. Bioorg. Med. Chem. 2011, 19, 7252.
[37] Dasari, R.; Banuls, L. M.; Masi, M.; Pelly, S. C.; Mathieu, V.; Green, I. R.; Otterlo, W. A. L.; Evidente, A.; Kiss, R.; Kornienko, A. Bioorg. Med. Chem. Lett. 2014, 24, 923.
[38] Giordani, R. B.; Junior, C. O. R.; Andrade, J. P.; Bastida, J.; Zuanazzi, J. A.; Tasca, T.; Almeida, M. V.; Chem. Biol. Drug Des. 2012, 80, 129.
[39] Lamora-Theys, D.; Andolfi, A.; Goietsenoven, G. V.; Cimmino, A.; Calve, B. L.; Wauthoz, N.; Megalizzi, V.; Gras, T.; Bruyere, C.; Dubois, J.; Mathieu, V.; Kornienko, A.; Kiss, R.; Evidente, A. J. Med. Chem. 2009, 52, 6244.
[40] Munulty, J.; Nair, J.; Little, J.; Brennan, J.; Bastida, J. Bioorg. Med. Chem. Lett. 2010, 20, 5290.
[41] Toriizuka, Y.; Kinoshita, E.; Kogure, N.; Kitajima, M.; Ishiyama, A.; Otoguro, K.; Yamada, H.; Omura, S.; Takayama, H. Bioorg. Med. Chem. 2008, 16, 10182.
[42] Evidente, A.; Cicala, M. R.; Randazzo, G.; Riccio, R.; Calabrese, G.; Liso, R.; Arrigoni, O. Phytochemisry 1983, 22, 2193.
[43] Huang, W. J.; Singh, O. V.; Chen, C. H.; Chiou, S. Y.; Lee, S. S. Helv. Chim. Acta 2002, 85, 1069.
[44] Cedron, J. C.; Gutierrez, D.; Plores, N.; Ravelo, A. C.; Estevez, B. A. Bioorg. Med. Chem. 2010, 18, 4694.
[45] Lee, S. S.; Venkatesham, U.; Rao, C. P. R.; Lam, S. H.; Lin, J. H. Bioorg. Med. Chem. 2007, 15 1034.
/
| 〈 |
|
〉 |