

铜催化下苯并五元杂环化合物的C—S偶联反应
收稿日期: 2014-10-09
修回日期: 2014-12-03
网络出版日期: 2014-12-10
基金资助
山西省科技基础条件平台建设(No. 2013091026)、山西大学本科生科研训练(No. 2014013)资助项目.
C—S Coupling Reaction of Heteroaromatic Compounds via Copper Catalyst
Received date: 2014-10-09
Revised date: 2014-12-03
Online published: 2014-12-10
Supported by
Project supported by the Science and Technology Basic Conditions Platform Construction Project of Shanxi Province (No. 2013091026) and the Provincial Training Program of Innovation and Entrepreneurship for Undergraduates (No. 2014013).
张变香 , 陈凯 , 杨丽花 , 许钰涛 , 张瑞杰 , 张利娜 , 史瑞雪 . 铜催化下苯并五元杂环化合物的C—S偶联反应[J]. 有机化学, 2015 , 35(4) : 905 -909 . DOI: 10.6023/cjoc201410009
In this study, the direct copper-catalyzed C—S coupling reaction of heterocyclic compounds with diphenyl disulfide, without the existence of ligands and alkalis, has been developed. It showed that the yield of target product was 89.9% when the reaction was carried out at 110 ℃, the reaction time was 12 h, dimethylsulfoxide (DMSO) was used as solvents, and the molar ratio of heterocyclic compound to biphenyl disulfide was 2:1. The structures of the target products were confirmed with 1H NMR and 13C NMR. The effects of various conditions on the reaction were investigated by high performance liquid chromatography (HPLC) analysis and its reaction mechanism was discussed.
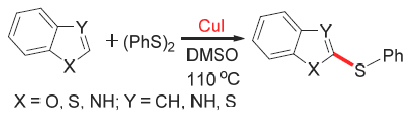
Key words: aryl thioether; benzo heterocyclic compounds; diphenyl disulfide; HPLC
[1] (a) Liu, G.; Huth, J. R.; Olejniczak, E. T.; Mendoza, R.; DeVries, P.; Leitza, S.; Reilly, E. B.; Okasinski, G. F.; Fesik, S. W.; von, Geldern, T. W. J. Med. Chem. 2001, 44, 1202.
(b) Gangjee, A.; Zeng, Y. B.; Talreja, T.; McGuire, J. J.; Kisliuk, R. L.; Queener, S. F. J. Med. Chem. 2007, 50, 3046.
(c) Liu, G.; Link, J. T.; Pei, Z.; Reilly, E. B.; Leitza, S.; Nguyen, B.; Marsh, K. C.; Okasinski, G. F.; von, Geldern, T. W.; Ormes, M.; Fowler, K.; Gallatin, M. J. Med. Chem. 2000, 43, 4025.
(d) Beard, R. L.; Colon, D. F.; Song, T. K.; Davies, P. J.; Kochhar, D. M.; Chandraratna, R. A. J. Med. Chem. 1996, 39, 3556.
(e) Martino, G. D.; Edler, M. C.; Regina, G. L.; Coluccia, A.; Barbera, M. C.; Barrow, D.; Nicholson, R. I.; Chiosis, G.; Brancale, A.; Hamel, E.; Artico, M.; Silvestri, R. J. Med. Chem. 2006, 49, 947.
(f) Rakitin, O. A. Sci. Synth. 2007, 31, 975.
(g) Chen, H. Y.; Peng, W. T.; Lee, Y. H. Organometallics 2013, 32, 5514.
[2] Song, H.; Leninger, M.; Lee, N.; Liu, P. H. Org. Lett. 2013, 15, 4854.
[3] Yang, M.; Pei, J.; Yan, G.; Weng, Q. Chin. J. Org. Chem. 2013, 33, 343 (in Chinese).
(杨明华, 裴吉, 严国兵, 翁秋月, 有机化学, 2013, 33, 343.)
[4] Qin, Y.; Peng, Q. Chin. J. Org. Chem. 2011, 31, 1169 (in Chinese).
(秦元成, 彭强, 有机化学, 2011, 31, 1169.)
[5] Liu, K.; Ou, H.; Shi, X.; Dong, X.; Ma, W.; Wei, J. Chin. J. Org. Chem. 2014, 34, 681 (in Chinese).
(刘课艳, 偶辉, 石先莹, 董雪芬, 马文娟, 魏俊发, 有机化学, 2014, 34, 681.)
[6] Huang, X.; Zhu, Q.; Xu, Y. Synth. Commun. 2001, 31, 2823.
[7] Mondal, J.; Borah, P.; Modak, A.; Zhao, Y. L.; Bhaumik, A. Org. Process Res. Dev. 2014, 18, 257.
[8] Varala, R.; Ramu, E.; Alam, M. M.; Adapa, S. R. Chem. Lett. 2004, 33, 1614.
[9] Pino, C. J.; Algarra, A. G.; Basallote, M. G. Inorg. Chem. 2013, 52, 14334.
[10] Qiao, Z. J.; Liu, H.; Xiao, X.; Fu, X. Org. Lett. 2013, 15, 2594.
[11] Zhang, X. Y.; Zeng, W. L.; Yang, Y.; Huang, H.; Liang, Y. Org. Lett. 2014, 16, 876.
[12] Krief, A.; Dumont, W.; Robert, M. Synlett 2006, 484.
[13] Wang, B.; Graskemper, J. W.; Qin, L.; DiMagno, S. G. Angew. Chem., Int. Ed. 2010, 49, 4079.
[14] Azeredo, J. B.; Godoi, M.; Martins, G. M.; Silveira, C. C.; Braga, A. L. J. Org. Chem. 2014, 79, 4125.
[15] Kumar, P. P.; Reddy, Y. D.; Reddy, C. V.; Devi, B. R.; Dubey, P. K. J. Sulfur Chem. 2014, 35, 356.
[16] Yang, F. L.; Tian, S. K. Angew. Chem., Int. Ed. 2013, 52, 4929.
[17] Ajiki, K.; Hirano, M.; Tanaka, K. Org. Lett. 2005, 7, 4193.
[18] Kumat, S.; Engman, L. J. Org. Chem. 2006, 71, 5400.
[19] Li, Z.; Hong, J. Q.; Zhou, X. G. Tetrahedron 2011, 67, 3690.
[20] Luo, P.; Yu, M.; Tang, R.; Zhong, P.; Li, J. Tetrahedron Lett. 2009, 50, 1066.
[21] Ge, W.; Wei, Y. Green Chem. 2012, 14, 2066.
[22] Zhang, B. X.; Chang, J.; Yang, Q.; Wu, Q. Chin. J. Org. Chem. 2012, 32, 1150 (in Chinese).
(张变香, 常姣, 杨祺, 吴群, 有机化学, 2012, 32, 1150.)
[23] Fukuzawa, S.; Shimizu, E.; Atsuumi, Y.; Haga, M.; Ogata, K. Tetrahedron Lett. 2009, 50, 2374.
[24] Nobukazu, T. J. Org. Chem. 2006, 71, 7874.
[25] Deshmukh, K. M.; Madyal, R. S.; Qureshi, Z. S.; Gaikar, V. G.; Bhanage, B. M. Ind. Eng. Chem. Res. 2013, 52, 4747.
[26] (a) Silveira, C. C.; Mendes, S. R.; Wolf, L.; Martins, G. M. Tetrahedron Lett. 2010, 51, 2014.
(b) Fang, X. L.; Tang, R. Y.; Zhong, P.; Li, J. H. Synthesis 2009, 24, 4183.
(c) Chen, Y.; Cho, C. H.; Shi, F.; Larock, R. C. J. Org. Chem. 2009, 74, 6802.
[27] Allin, S. M.; Bowman, W. R.; Karim, R.; Rahman, S. S. Tetrahedron 2006, 62, 4306.
/
| 〈 |
|
〉 |