

邻氨基芳香腈与羰基化合物的反应机理及其产物的骨架结构
收稿日期: 2014-06-06
修回日期: 2014-07-16
网络出版日期: 2014-08-26
基金资助
北京理工大学基础研究基金(No.2012CX100035)资助项目.
Structure of the Condensed Product of Aromatic o-Aminonitrile with Carbonyl Compound and Its Mechanism
Received date: 2014-06-06
Revised date: 2014-07-16
Online published: 2014-08-26
Supported by
Project supported by the Basic Research Fund of Beijing Institute of Technology (No. 2012CX100035).
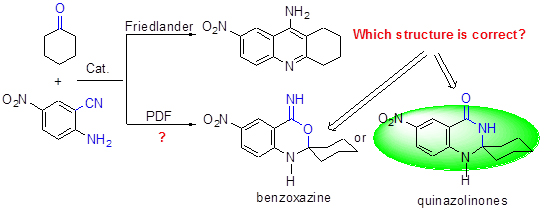
Friedländer反应是合成具有生物活性、光电活性的喹啉衍生物的重要方法. 其典型转化之一——邻氨基芳香腈与羰基化合物的Friedländer缩合在得到经典的转化产物的同时可以得到新骨架产物, 这种新转化自本课题组报道以来已受到国内外学者的诸多关注, 但该转化的新产物骨架结构尚存争议. 本综述概括了该新转化自发现以来国内外的研究进展, 从13C NMR, FT-IR, X射线单晶衍射数据等明确了新转化反应得到的产物骨架为喹唑啉酮结构. 完整地提出了该类Friedländer分岔反应的转化机理: 经由分子内的Pinner反应到Dimroth重排的过程存在于邻氨基芳香腈和酮的经典Friedländer反应中(PDF反应).
杨俊娟 , 史大昕 , 刘明星 , 张立军 , 张奇 , 李加荣 . 邻氨基芳香腈与羰基化合物的反应机理及其产物的骨架结构[J]. 有机化学, 2014 , 34(12) : 2424 -2437 . DOI: 10.6023/cjoc201406007
Friedländer reaction is one of the most important routes to synthesize the quinoline and its derivatives, which possesses excellent bioactivity and photo-electricity activity. Recently, a new skeleton product besides the normal Friedländer quinoline was discovered by the condensation of aromatic o-aminonitrile and carbonyl compounds, and this phenomenon has attracted many researchers, but the skeleton structure of new conversion is debated. Herein, this paper reviews on the development progress of this new kind reaction. According to recent research results, the skeleton structure of new conversion was assigned as quinazolinone, which was confirmed by the 13C NMR, FT-IR, and single-crystal X-ray diffraction deterimations. Therefore, this new conversion is abbreviated as PDF conversion, which means a new conversion from Pinner to Dimroth rearrangement in the Friedländer reaction. The total reaction mechanism of o-aminonitrile with carbonyl compound was proposed.

[1] Richter, M.; Molnár, J.; Hilgeroth, A. J. Med. Chem. 2006, 49, 2838.
[2] Elnagdi, M. H.; Erian, A. W. W. Arch. Pharm. 1991, 324, 853.
[3] Ferris, J. P.; Orgel, L. E. J. Am. Chem. Soc. 1966, 88, 3829.
[4] Dias, A. M.; Cabral, I.; Proença, M. F.; Booth, B. L. J. Org. Chem. 2002, 67, 5546.
[5] Taylor, E. C.; Wachsen, E. J. Org. Chem. 1978, 43, 4154.
[6] Erian, A. W. Chem. Rev. 1993, 93, 1991.
[7] Abdelrazek, F. M.; Bahbouh, M. S.; Jordan, J. Earth Environ. Sci. 2012, 4, 47.
[8] Camps, P.; Gómez, E.; Muñoz, Torrero, D.; Badia, A.; Vivas, N. M.; Barril, X.; Orozco, M.; Luque, F. J. J. Med. Chem. 2001, 44, 4733.
[9] McKenna, M. T.; Proctor, G. R.; Young, L. C.; Harvey, A. L. J. Med. Chem. 1997, 40, 3516.
[10] Barreiro, E. J.; Camara, C. A.; Verli, H.; Brazil, Mas, L.; Castro, N. G.; Cintra, W. M.; Aracava, Y.; Rodrigues, C. R.; Fraga, C. A. J. Med. Chem. 2003, 46, 1144.
[11] Li, J.-R.; Ma, S.-L. Chin. Chem. Lett. 2005, 16, 1424 (in Chinese). (李加荣, 马淑玲, 中国化学快报, 2005, 16, 1424.)
[12] Zhen, B.; Jiao, Q.; Zhang, Y.; Wu, Q.; Li, H. S.; Shi, D. X.; Li, J. R. Catal. Commun. 2013, 32, 1.
[13] Yang, L.-P.; Li, J.-R.; Chai, H.-X.; Yuan, H.; Zhang, Q.; Shi, D.-X. Chin. J. Org. Chem. 2013, 33, 174 (in Chinese). (杨留攀, 李加荣, 柴红新, 袁洪, 张奇, 史大昕, 有机化学, 2013, 33, 174.)
[14] Yang, D. L.; Shi, D. X.; Zhang, Q.; Chai, H. X.; Li, J. R. Acta Crystallogr., Sect. B: Struct. Sci. 2013, 69, o633.
[15] Yang, D.-L.; Li, J.-R.; Sun, K.-N.; Lu, H.-Y.; Liu, M.-X.; Shi, D.-X. Chin. J. Org. Chem. 2013, 33, 2341 (in Chinese). (杨德利, 李加荣, 孙克宁, 路红燕, 刘明星, 史大昕, 有机化学, 2013, 33, 2341.)
[16] Yang, L. P.; Shi, D. X.; Chen, S.; Chai, H. X.; Huang, D. F.; Zhang, Q.; Li, J. R. Green Chem. 2012, 14, 945.
[17] Tang, J. H.; Li, J. R.; Zhang, L.; Ma, S. L.; Shi, D. X.; Zhang, Q.; Yang, L. P.; Wang, X.; Liu, X.; Liu, C. J. Heterocycl. Chem. 2012, 49, 533.
[18] Liu, C.-E.; Tang, J.-H.; Li, J.-R. Chin. J. Org. Chem. 2012, 32, 532 (in Chinese). (刘长娥, 唐建红, 李加荣, 有机化学, 2012, 32, 532.)
[19] Liu, X.; Shi, D. X.; Tang, J. H.; Yang, D. L. Acta Crystallogr., Sect. E: Struct. Sci. 2011, 67, o2016.
[20] Tang, J. H.; Shi, D. X.; Yan, L. P.; Liu, X.; Li, J. R. Acta Crystallogr., Sect. B: Struct. Sci. 2011, 67, o1672.
[21] Li, J. R.; Chen, X.; Shi, D. X.; Ma, S. L.; Li, Q.; Zhang, Q.; Tang, J. H. Org. Lett. 2009, 11, 1193.
[22] Li, J. R.; Zhang, L.; Shi, D. X.; Li, Q.; Wang, D.; Wang, C.; Zhang, Q.; Fan, Y. Synlett 2008, 233.
[23] Wu, X. F.; Oschatz, S.; Block, A.; Spannenberg, A.; Langer, P. Org. Biomol. Chem. 2014, 12, 1865.
[24] Gyuris, M.; Puskas, L. G.; Toth, G. K.; Kanizsai, I. Org. Biomol. Chem. 2013, 11, 6320.
[25] Kanawade, S. B.; Patil, S. P.; Nikam, P. S.; Gangurde, S. A.; Jachak, M. N.; Toche, R. B. J. Heterocycl. Chem. 2012, 49, 363.
[26] Majumdar, K. C.; Roy, B.; Debnath, P.; Taher, A. Curr. Org. Chem. 2010, 14, 846.
[27] Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; Carreiras, M. C.; Soriano, E. Chem. Rev. 2009, 109, 2652.
[28] Shaban, M. E.; Youssef, A. M.; Elaasar, N. K.; EI-Ziaty, A. K. Pak J. Sci. Ind. Res. 2008, 51, 119.
[29] Kouznetsov, V. V.; Mendez, L. Y.; Gomez, C. M. Curr. Org. Chem. 2005, 9, 141.
[30] Riesgo, E. C.; Jin, X.; Thummel, R. P. J. Org. Chem. 1996, 61, 3017.
[31] Yang, D.; Jiang, K.; Li, J.; Xu, F. Tetrahedron 2007, 63, 7654.
[32] Palimkar, S. S.; Siddiqui, S. A.; Daniel, T.; Lahoti, R. J.; Srinivasan, K. V. J. Org. Chem. 2003, 68, 9371.
[33] Dormer, P. G.; Eng, K. K.; Farr, R. N.; Humphrey, G. R.; McWilliams, J. C.; Reider, P.; Sager, J. W.; Volante, R. J. Org. Chem. 2003, 68, 467.
[34] Cheng, C. C.; Yan, S. J. Org. React. 1982, 28.
[35] Muchowski, J. M.; Maddox, M. L. Can. J. Chem. 2004, 82, 461.
[36] Leon, R.; Garcia, A. G.; Marco-Contelles, J. J. Chem. Res. 2006, 8, 536.
[37] Rane, B. S.; Kazi, M. A.; Bagul, S. M.; Shelar, D. P.; Toche, R. B.; Jachak, M. N. J. Fluoresc. 2010, 20, 415.
[38] Perez-Mayoral, E.; Musilova, Z.; Gil, B.; Marszalek, B.; Polozij, M.; Nachtigall, P.; Cejka, J. Dalton Trans. 2012, 41, 4036.
[39] Zhang, L. J.; Yu, J. L.; Wang, W. L.; Li, H.; Xu, D. D.; Bi, Y. D.; Liu, F. D. Tetrahedron Lett. 2014, 55, 710.
[40] Safari, J.; Gandomi-Ravandi, S. C. R. Chim. 2013, 16, 1158.
[41] Nguyen, T. B.; Ermolenko, L.; Al-Mourabit, A. Green Chem. 2013, 15, 2713.
[42] Labade, V. B.; Shinde, P. V.; Shingare, M. S. Tetrahedron Lett. 2013, 54, 5778.
[43] Desroses, M.; Scobie, M.; Helleday, T. New J. Chem. 2013, 37, 3595.
[44] Sharma, R.; Pandey, A. K.; Chauhan, P. M. S. Synlett 2012, 23, 2209.
[45] Ghashang, M. Orient. J. Chem. 2012, 28, 1213.
[46] Spagnol, G.; Rajca, A.; Rajca, S. J. Org. Chem. 2007, 72, 1867.
[47] Bonne, D.; Dekhane, M.; Zhu, J. Org. Lett. 2005, 7, 5285.
[48] Klemm, L. H.; Weakley, T. J. R.; Gilbertson, R. D.; Song, Y. H. J. Heterocycl. Chem. 1998, 35, 1269.
[49] Zhang, L. J.; Li, J. R.; Shi, D. X.; Chen, J. Acta Crystallogr., Sect. E: Struct. Sci. 2008, 64, o449.
[50] Zhang. L.; Shi, D. X.; Li, J. R.; Zhang, L.; Fan, Y. Q. Acta Crystallogr., Sect. E: Struct. Sci. 2008, 64, o1056.
[51] Zhang, L.; Shi, D. X.; Fan, Y. Q.; Qian, D.; Li, J. R. Acta Crystallogr., Sect. E: Struct. Sci. 2009, 65, o1345
[52] Zhang, L.; Li, J. R.; Yang, X.; Shi, D. X.; Chen, J. Acta Crystallogr., Sect. E: Struct. Sci. 2008, 64, o450.
[53] Zhang, L. J.; Li, J. R.; Shi, D. X.; Zhang, L.; Fan, Y. Q. Acta Crystallogr., Sect. E: Struct. Sci. 2008, 64, o448.
[54] Li, J.-R.; Zhang, L.-J.; Shi, D.-X.; Zhang, Q. Chin. Chem. Lett. 2008, 19, 15 (in Chinese). (李加荣, 张立军, 史大昕, 张奇, 中国化学快报, 2008, 19, 15.)
[55] Chao, X.; He, X.; Yang, Y.; Zhou, X.; Jin, M.; Liu, S.; Cheng, Z.; Liu, P.; Wang, Y.; Yu, J.; Tan, Y.; Huang, Y.; Qin, J.; Rapposelli, S.; Pi, R. Bioorg. Med. Chem. Lett. 2012, 22, 6498.
[56] Gutschow, M.; Neumann, U.; Sieler, J.; Eger, K. Pharm. Acta Helv. 1998, 73, 95.
[57] Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N. L. Angew. Chem., Int. Ed. 1995, 34, 1555.
[58] Etter, M. C.; MacDonald, J. C.; Bernstein, J. Acta Crystallogr., Sect. E: Struct. Sci. 1990, 46, 256.
[59] Li, J. R.; Ma, S. L.; Sun, Y. J.; Wei, X. J.; Zhou, Z. M. J. Heterocycl. Chem. 2006, 43, 745.
[60] Li, J.-R.; Sun, Y.-J.; Zhou, Z.-M. Chin. J. Org. Chem. 2006, 26, 928 (in Chinese). (李加荣, 马淑玲, 孙永江, 周智明, 有机化学, 2006, 26, 928.)
[61] Shi, D.; Dou, G.; Zhou, Y. Synthesis-Stuttgart 2008, 13, 2000.
[62] Shi, D. Q.; Rong, L. C.; Wang, J. X.; Zhuang, Q. Y.; Wang, X. S.; Hua, H. W. Tetrahedron Lett. 2003, 44, 3199.
[63] Cheng, X.; Vellalath, S.; Goddard, R.; List, B. J. Am. Chem. Soc. 2008, 130, 15786.
[64] Chinigo, G. M.; Paige, M.; Grindrod, S.; Hamel, E.; Dakshanamurthy, S.; Chruszcz, M.; Minor, W.; Brown, M. L. J. Med. Chem. 2008, 51, 4620.
[65] Diwu, Z.; Lu, Y.; Upson, R. H.; Zhou, M.; Klaubert, D. H.; Haugland, R. P. Tetrahedron 1997, 53, 7159.
[66] Zappalà, M.; Grasso, S.; Micale, N.; Zuccalà, G.; Menniti, F. S.; Ferreri, G.; De, S. G.; De, M. C. Bioorg. Med. Chem. Lett. 2003, 13, 4427.
[67] Mhaske, S. B.; Argade, N. P. Tetrahedron 2006, 62, 9787.
[68] He, F.; Snider, B. B. J. Org. Chem. 1999, 64, 1397.
[69] Siskos, A. P.; Hill, A. M. Tetrahedron Lett. 2003, 44, 789.
[70] Zaki, M. Molecules 1998, 3, 71.
[71] Elkholy, Y. M.; Morsy, M. A. Molecules 2006, 11, 890.
[72] Angelin, M.; Vongvilai, P.; Fischer, A.; Ramström, O. Chem. Commun. 2008, 6, 768.
[73] Angelin, M.; Fischer, A.; Ramström, O. J. Org. Chem. 2008, 73, 3593.
[74] Lajoie, G.; Lepine, F.; Maziak, L.; Belleau, B. Tetrahedron Lett. 1983, 24, 3815.
[75] Neugebauer, W.; Pinet, E.; Kim, M.; Carey, P. R Can. J. Chem. 1996, 74, 341.
[76] Luzyanin, K. V.; Kukushkin, V. Y.; Kuznetsov, M. L.; Garnovskii, D. A.; Haukka, M.; Pombeiro, A. J. Inorg. Chem. 2002, 41, 2981.
[77] Wang, J.; Wang, J.; Zhu, Y.; Lu, P.; Wang, Y. Chem. Commun. 2011, 47, 3275.
[78] Lin, G.-C.; Liu, L.; Zhang, L.- R.; Zhang, L.-H. Chin. J. Inorg. Chem. 2002, 10, 405 (in Chinese). (林桂椿, 刘莉, 张亮仁, 张礼和, 合成化学, 2002, 10, 405.)
[79] Kurz, T.; Widyan, K.; Wackendorff, C.; Schlüter, K. Synthesis 2004, 1987.
[80] Liu, X.; Shi, D.-X.; Tang, J.-H.; Zhang, Q.; Li, J.-R. Chin. J. Org. Chem. 2011, 31, 1710 (in Chinese). (刘璇, 史大昕, 唐建红, 张奇, 李加荣, 有机化学, 2011, 31, 1710.)
[81] Zhang, P. W.; Terefenko, E. A.; Fensome, A.; Wrobel, J.; Winneker, R.; Lundeen, S.; Marschke, K. B.; Zhang, Z. M. J. Med. Chem. 2002, 45, 4379.
[82] Kamal, A.; Ramana, K. V.; Rao, M. V. J. Org. Chem. 2001, 66, 997.
[83] Yang, L. P.; Li, J. R.; Chai, H. X.; Lu, H. Y.; Zhang, Q.; Shi, D. X. Chin. J. Chem. 2013, 31, 443.
/
| 〈 |
|
〉 |