

新型5,6,7,8-四氢苯并噻吩并嘧啶酮衍生物的合成与抗肿瘤活性研究
收稿日期: 2014-10-24
修回日期: 2015-12-15
网络出版日期: 2015-01-05
基金资助
湖北自然科学基金(No.2011CDC006)、湖北省2011协同创新中心基金(No.2011JH-2014CXTT07)和湖北医药学院优秀中青年科技创新团队研究(Nos.2011CXX03,2009QDJ15,2014XKJSXJ06)资助项目.
Synthesis and Antitumor Activity of Some Novel 5,6,7,8-Tetrahydro- benzo-thieno[2,3-d]pyrimidin-4(3H)-one Derivatives
Received date: 2014-10-24
Revised date: 2015-12-15
Online published: 2015-01-05
Supported by
Project supported by the Natural Science Foundation of Hubei Province (No.2011CDC006), the Fund of Hubei 2011 Cooperative Innovation Center (No.2011JH-2014CXTT07) and the Science and Technology Innovation team Project of Hubei University of Medicine (Nos.2011CXX03, 2009QDJ15, 2014XKJSXJ06).
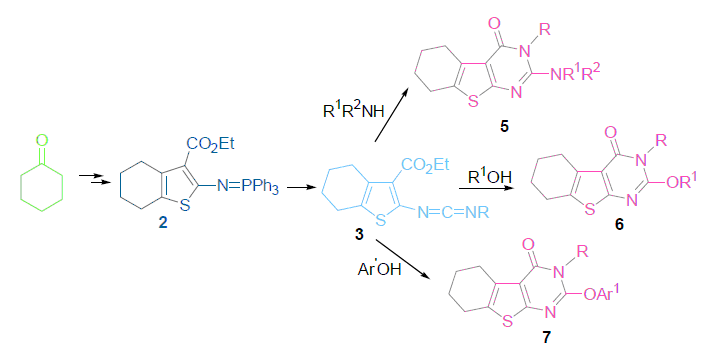
应用5,6,7,8-四氢苯并噻吩膦亚胺(2)与烷基异氰酸酯的氮杂Wittig反应生成碳二亚胺2, 在不同的条件下, 2分别与不同的亲核试剂反应, 有效地合成了不同取代的新型稠合噻吩并[2,3-d]嘧啶-4(3H)-酮衍生物. 所得化合物的结构由1H NMR, IR, MS和元素分析所确证. 初步的体外抗肿瘤活性测试, 结果显示目标化合物具有抑制肿瘤细胞的生长, 其中5d对口腔肿瘤细胞KB2IC50值为19.0 μmol/L, 表现出潜在的抗肿瘤活性.
关键词: 氮杂Wittig反应; 噻吩并嘧啶酮; 合成; 抗肿瘤活性
王红梅 , 郭树兵 , 胡扬根 , 曾小华 , 杨光义 . 新型5,6,7,8-四氢苯并噻吩并嘧啶酮衍生物的合成与抗肿瘤活性研究[J]. 有机化学, 2015 , 35(5) : 1075 -1080 . DOI: 10.6023/cjoc201410035
The aza-Wittig reaction of iminophosphorane 2 with alkyl isocyanates gave carbodiimides 3, which were allowed to react with various nucleophilic reagents under mild condition in satisfactory yields to prepare a novel series of 5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidin-4(3H)-one derivatives. The structures of these compounds were confirmed by 1H NMR, IR, MS and elemental analysis. The in vitro antitumor activities of compounds were analyzed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-liumbromide (MTT) standard method, 5d stood out as the most potent showing an IC50 of 19.0 μmol/L against human tumor cell lines (KB).

[1] (a) Walter, H. WO 99/14202, 1999 [Chem. Abstr. 1999, 130, 252368k]. (b) Walter, H. WO 0034286, 2000 [Chem. Abstr. 2000, 133, 30738d].
[2] (a) Demirayak, S.; Mohsen, U. A. Heterocycl. Chem. 2001, 38, 507. (b) Santagati, A.; Santagati, M.; Modica, M. Heterocycles 1993, 36, 1315.
[3] Jennings, L. D.; Kincaid, S. L.; Wang, Y. D.; Krishnamurthy, G.; Beyer, C. F.; McGinnis, J. P.; Miranda, M.; Discafani, C. M.; Rabindran, S. K. Bioorg. Med. Chem. Lett. 2005, 15, 4731.
[4] Duval, E.; Case, A.; Stein, R. L.; Cuny, G. D. Bioorg. Med. Chem. Lett. 2005, 15, 1885.
[5] Perspicace, E.; Jouan-Hureau, V.; Ragno, R.; Ballante, F.; Sartini, S.; La Motta, C.; Da Settimo, F.; Chen, B.; Kirsch, G.; Schneider, S.; Faivr, B.; Hesse, S. Eur. J. Med. Chem. 2013, 63, 765.
[6] Wu, C.-H.; Coumar, M. S,; Chu, C.-Y.; Lin, W.-H.; Chen, Y.-R.; Chen, C.-T.; Shiao, H.-Y.; Rafi, S.; Wang, S.-Y.; Hsu, H.; Chen, C.-H.; Chang, C.-Y.; Chang, T.-Y.; Lien, T.-W.; Fang, M.-Y.; Yeh, T.-K.; Hsieh, S.-H.; Hsu, J. T.-A.; Liao, C.-C.; Chao, Y.-S.; Hsieh, H.-P. J. Med. Chem. 2010, 53, 7316.
[7] Zeng, G.; Zheng, P. Acta Chim. Sinica 2012. 70(6), 759.
[8] Hu, Y.-G.; Wang, Y.; Du, S.-M.; Chen, X.-B.; Ding, M.-W. Bioorg. Med. Chem. Lett. 2010, 20, 6188.
[9] Hu, Y. G.; Lu, M. Y.; Song, H. L.; Ding, M. W. Chin. J. Org. Chem. 2005, 25, 295 (in Chinese). (胡扬根, 吕茂云, 宋鹤丽, 丁明武, 有机化学, 2005, 25, 295.)
[10] Hu, Y. G.; Gao, H.-T.; Wang, G.; Wang, Y.; Qu, Y. N.; Xu, J. Chin. J. Org. Chem. 2012, 32, 1468 (in Chinese). (胡扬根, 高海涛, 王刚, 王燕, 屈永年, 徐靖, 有机化学, 2012, 32, 1468.)
[11] Hu, Y.-G.; Xu, J.; Chen, X.-B.; Qu, Y.-N. Chin. J. Org. Chem. 2009, 29, 1853 (in Chinese). (胡扬根, 徐靖, 陈小保, 屈永年, 有机化学, 2009, 29, 1853.)
[12] Ding, M.-W.; Yang, S.-J.; Zhu, J. Synthesis 2004, 75.
[13] (a) Zeng, X.-H.; Wang, H.-M.; Cui, Z.-P.; Ding, M.-W.; He, H.-W. Acta Crystallogr. 2006, E62, o228. (b) Zeng, X.-H.; Yuan, J.-Z.; Huang, N.-Y.; Ding, M.-W.; He, H.-W. Acta Crystallogr. 2005. E61, o4336.
[14] Ding, M. W.; Huang, N. Y.; He, H. W. Synthesis 2005, 1601.
/
| 〈 |
|
〉 |