

含联苯醚结构2(5H)-呋喃酮衍生物的合成
收稿日期: 2014-11-05
修回日期: 2014-12-09
网络出版日期: 2014-01-05
基金资助
国家自然科学基金(No.31200439)、广东省高等学校人才引进专项资金(No.粤财教[2011]431号)、广东省自然科学基金(No.2014A030313429)资助项目.
Synthesis of 2(5H)-Furanone Derivatives with Biphenyl Ether Unit
Received date: 2014-11-05
Revised date: 2014-12-09
Online published: 2014-01-05
Supported by
Project supported by the National Natural Science Foundation of China (No.31200439), the 3rd Talents Special Funds of Guangdong Higher Education (No.Guangdong-Finance-Education [2011]431) and the Natural Science Foundation of Guangdong Province (No.2014A030313429).
关丽涛 , 莫广珍 , 吴彦城 , 梁欣榆 , 罗俏芳 , 汪朝阳 . 含联苯醚结构2(5H)-呋喃酮衍生物的合成[J]. 有机化学, 2015 , 35(5) : 1081 -1089 . DOI: 10.6023/cjoc201411006
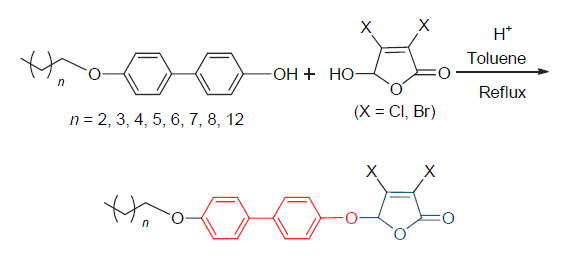
Sixteen 5-(4'-alkoxybiphenyl-4-yloxy)-3,4-dihalo-2(5H)-furanones are synthesized via the direct dehydrative etherification reactions of 4'-alkoxybiphenyl-4-ol with mucochloric acid and mucobromic acid, respectively, using sulfuric acid as catalyst at reflux in toluene from 30% to 58% yields (mostly over 47%). The moderate yields may be due to that this acid-catalyzed etherification should be a SN2 nucleophilic substitution reaction. The structures of all newly synthesized compounds are elucidated and confirmed by FTIR, UV, 1H NMR, 13C NMR, and elemental analysis. The synthetic procedures afford not only an important strategy for the synthesis of 2(5H)-furanone derivatives having potential bioactivity, but also a simple method for the synthesis of aromatic ether from two different hydroxyl compounds.
[1] (a) Trost, B. M.; Burns, A. C.; Bartlett, M. J.; Tautz, T.; Weiss, A. H. J. Am. Chem. Soc. 2012, 134, 1474. (b) Xie, Y.-Z.; Wang, N.; Cheng, B.; Zhai, H.-B. Org. Lett. 2012, 14, 3. (c) Xue, F.-L.; Li, J.-X.; Wang, Z.-Y.; Xiong, J.-F.; Li, D. Res. Chem. Intermed. 2013, 39, 1153. (d) Tatton, M. R.; Simpson, I.; Donohoe, T. J. Chem. Commun. 2014, 50, 11314.
[2] Guerrero, M. D.; Aquino, M.; Bruno, I.; Terencio, M. C.; Paya, M.; Riccio, R.; Gomez-Paloma, L. J. Med. Chem. 2007, 50, 2176.
[3] Wei, M.-X.; Feng, L.; Li, X.-Q.; Zhou, X.-Z.; Shao, Z.-H. Eur. J. Med. Chem. 2009, 44, 3340.
[4] (a) Kamal, A.; Suresh, P.; Mallareddy, A.; Kumar, B. A.; Reddy, P. V.; Raju, P.; Tamboli, J. R.; Shaik, T. B.; Jain, N.; Kalivendi, S. V. Bioorg. Med. Chem. 2011, 19, 2349. (b) Bromhead, L. J.; Visser, J.; McErlean, C. S. P. J. Org. Chem. 2013, 79, 1516. (c) Pereira, U. A.; Barbosa, L. C. A.; Maltha, C. R. A. Bioorg. Med. Chem. Lett. 2014, 24, 1052.
[5] Sarma, K. D.; Zhang, J.; Curran, T. T. J. Org. Chem. 2007, 72, 3311.
[6] (a) Jiang, Y.-Q.; Shi, Y.-L.; Shi, M. J. Am. Chem. Soc. 2008, 130, 7202. (b) Zhou, L.-H.; Yu, X.-Q.; Pu, L. J. Org. Chem. 2009, 74, 2013. (c) Perez, M.; Perez, D. I.; Martinez, A.; Castro, A.; Gomez, G.; Fall, Y. Chem. Commun. 2009, 3252. (d) Cui, H.-L.; Huang, J.-R.; Lei, J.; Wang, Z.-F.; Chen, S.; Chen, Y.-C. Org. Lett. 2010, 12, 720.
[7] (a) Tan, Y.-H.; Li, J.-X.; Xue, F.-L.; Qi, J.; Wang, Z.-Y. Tetrahedron 2012, 68, 2827. (b) Mo, Y.-Q.; Wang, Z.-Y.; Mei, W.-J.; Fu, J.-H.; Tan, Y.-H.; Luo, S.-H. Monatsh. Chem. 2012, 143, 443. (c) Huo, J.-P.; Lv, M.-X.; Wang, Z.-Y.; Li, Y.-Z. Chin. J. Chem. 2012, 30, 2411. (d) Huo, J.-P.; Xiong, J.-F.; Mo, G.-Z.; Peng, P.; Wang, Z.-Y. Res. Chem. Intermed. 2013, 39, 4321.
[8] Janody, S.; Hermange, P.; Retailleau, P.; Thierry, J.; Dodd, R. H. J. Org. Chem. 2014, 79, 5673.
[9] Latypova, L. Z.; Saigitbatalova, E. S.; Chulakova, D. R.; Lodochnikova, O. A.; Kurbangalieva, A. R.; Berdnikov, E. A.; Chmutova, G. A. Russ. J. Org. Chem. 2014, 50, 521.
[10] (a) Hajduk, P. J.; Sheppard, G.; Nettesheim, D. G.; Hajduk, P. J.; Sheppard, G.; Nettesheim, D. G.; Olejniczak, E. T.; Shuker, S. B.; Meadows, R. P.; Steinman, D. H.; Carrera, G. M.; Marcotte, P. A.; Severin, J.; Walter, K.; Smith, H.; Gubbins, E.; Simmer, R.; Holzman, T. F.; Morgan, D. W.; Davidsen, S. K.; Summers, J. B.; Fesik, S. W. J. Am. Chem. Soc. 1997, 119, 5818. (b) Kim, S. H.; Kim, J. G. Bull. Korean Chem. Soc. 2011, 32, 341. (c) Zhou, C.; Li, Y.-M.; Lu, Y.; Zhang, R.; Jin, K.; Fu, X.-M.; Duan, C.-Y. Chin. J. Chem. 2013, 31, 1269. (d) Hawksworth, E. L.; Andrews, P. C.; Lie, W.; Lai, B.; Dillon, C. T. J. Inorg. Biochem. 2014, 135, 28. (e) Belema, M.; Nguyen, V. N.; Bachand, C.; Deon, D. H.; Goodrich, J. T.; James, C. A.; Lavoie, R.; Lopez, O. D.; Martel, A.; Romine, J. L.; Ruediger, E. H.; Snyder, L. B.; St Laurent, D. R.; Yang, F.-K.; Zhu, J.-L.; Wong, H.-S.; Langley, D. R.; Adams, S. P.; Cantor, G. H.; Chimalakonda, A.; Fura, A.; Johnson, B. M.; Knipe, J. O.; Parker, D. D.; Santone, K. S.; Fridell, R. A.; Lemm, J. A.; O'Boyle, D. R.; Colonno, R. J.; Gao, M.; Meanwell, N. A.; Hamann, L. G. J. Med. Chem. 2014, 57, 2013.
[11] (a) Ortgies, D. H.; Forgione, P. Synlett 2013, 1715. (b) Ortgies, D. H.; Barthelme, A.; Aly, S.; Desharnais, B.; Rioux, S.; Forgione, P. Synthesis 2013, 694. (c) Costa, N. E.; Pelotte, A. L.; Simard, J. M. J. Chem. Educ. 2012, 89, 1064. (d) Wang, L.-M.; Cheng, S.-X.; Chen, T.; Chang, J.-B. Acta Chim. Sinica 2012, 70, 1201 (in Chinese). (王利敏, 程森祥, 陈彤, 常俊标, 化学学报, 2012, 70, 1201.) (e) Jiang, G.-F.; Niu, P.-P.; Zhang, Z.-C.; Yan, B.-M.; Guo, C.-C. J. Hunan Univ. (Nat. Sci. Ed.) 2014, 41, 112 (in Chinese). (江国防, 牛平平, 张志超, 焉保明, 郭灿城, 湖南大学学报(自然科学版), 2014, 41, 112.) (f) Bernaskova, M.; Kretschmer, N.; Schuehly, W.; Huefner, A.; Weis, R.; Bauer, R. Molecules 2014, 19, 1223.
[12] (a) Jauch, J. J. Org. Chem. 2001, 66, 609. (b) Lattmann, E.; Dunn, S.; Niamsanit, S.; Sattayasai, N. Bioorg. Med. Chem. Lett. 2005, 15, 919. (c) Song, Y. S.; Lee, Y.-J.; Kim, B. T.; Heo, J.-N. Tetrahedron Lett. 2006, 47, 7427. (d) Boukouvalas, J.; Côté, S.; Ndzi, B. Tetrahedron Lett. 2007, 48, 105. (e) Bellina, F.; Marchetti, C.; Rossi, R. Eur. J. Org. Chem. 2009, 4685. (f) Banwell, M. G.; Jones, M. T.; Loong, D. T. J.; Lupton, D. W.; Pinkerton, D. M.; Ray, J. K.; Willis, A. C. Tetrahedron 2010, 66, 9252. (g) Harb, H. Y.; Collins, K. D.; Altur, J. V. G.; Bowker, S.; Campbell, L.; Procter, D. J. Org. Lett. 2010, 12, 5446. (h) Fu, J.-H.; Wang, Z.-Y.; Huo, J.-P.; Tan, Y.-H.; Zeng, R.-H. Chin. J. Org. Chem. 2012, 32, 104 (in Chinese). (傅建花, 汪朝阳, 霍景沛, 谭越河, 曾荣华, 有机化学, 2012, 32, 104.) (i) Chen, R.-H.; Fu, J.-H.; Li, G.-L.; Tan, Y.-H.; Wang, Z.-Y.; Yuan, P. Chin. J. Org. Chem. 2012, 32, 95 (in Chinese). (陈任宏, 傅建花, 李国良, 谭越河, 汪朝阳, 袁萍, 有机化学, 2012, 32, 95.) (j) Xue, F.-L.; Li, J.-X.; Mo, Y.-Q.; Wang, Z.-Y.; Chen, Q.-H. Chin. J. Org. Chem. 2012, 32, 284 (in Chinese). (薛福玲, 李建晓, 莫阳青, 汪朝阳, 陈庆华, 有机化学, 2012, 32, 284.)
[13] (a) Polezhaeva, N. A.; Kalinina, I. V.; Volodina, Y. M.; Berdnikov, E. A.; Galkin, V. I.; Cherkasov, R. A. Russ. J. Gen. Chem. 2004, 40, 438. (b) Zhang, J.; Blazecka, P. G.; Curran, T. T. Tetrahedron Lett. 2007, 48, 2611. (c) Liu, G.-Y.; Guo, B.-Q.; Chen, W.-N.; Cheng, C.; Zhang, Q.-L.; Dai, M.-B.; Sun, J.-R.; Sun, P.-H.; Chen, W.-M. Chem. Biol. Drug Des. 2012, 79, 628. (d) Claraz, A.; Oudeyer, S.; Levacher, V. Adv. Synth. Catal. 2013, 355, 841.
[14] (a) Yadav, G. D.; Desai, N. M. Catal. Commun. 2006, 7, 325. (b) Haibach, M. C.; Guan, C. J.; Wang, D. Y.; Li, B.; Lease, N.; Steffens, A. M.; Karsten, K. J.; Goldman, A. S. J. Am. Chem. Soc. 2013, 135, 15062. (c) Zang, Y.; Ojima, I. J. Org. Chem. 2013, 78, 4013. (d) Ravi, K. G. S.; Chang, C.-W.; Chein, R.-J. Tetrahedron Lett. 2014, 55, 1031.
[15] Murai, M.; Origuchi, K.; Takai, K. Org. Lett. 2014, 16, 3828.
[16] (a) Huo, J.-P.; Luo, J.-C.; Wu, W.; Xiong, J.-F.; Mo, G.-Z.; Wang, Z.-Y. Ind. Eng. Chem. Res. 2013, 52, 11850. (b) Huo, J.-P.; Deng, G.-H.; Wu, W.; Xiong, J.-F.; Zhong, M.-L.; Wang, Z.-Y. Macromol. Rapid Commun. 2013, 34, 1779. (c) Xue, F.-L.; Qi, J.; Peng, P.; Mo, G.-Z.; Wang, Z.-Y. Lett. Org. Chem. 2014, 11, 64. (d) Xue, F.-L.; Peng, P.; Shi, J.; Zhong, M.-L.; Wang, Z.-Y. Synth. Commun. 2014, 44, 1944. (e) Tan, Y.-H.; Li, J.-X.; Huo, J.-P.; Xue, F.-L.; Wang, Z.-Y. Synth. Commun. 2014, 44, 2974.
[17] (a) Li, J.-X.; Xue, F.-L.; Tan, Y.-H.; Luo, S.-H.; Wang, Z.-Y. Acta Chim. Sinica 2011, 69, 1688 (in Chinese). (李建晓, 薛福玲, 谭越河, 罗时荷, 汪朝阳, 化学学报, 2011, 69, 1688.) (b) Li, J.-X.; Wang, Z.-Y.; Xue, F.-L.; Luo, S.-H. Acta Chim. Sinica 2011, 69, 2835 (in Chinese). (李建晓, 汪朝阳, 薛福玲, 罗时荷, 化学学报, 2011, 69, 2835.)
[18] Tang, B.-X.; Zhang, Y.-H.; Song, R.-J.; Tang, D.-J.; Deng, G.-B.; Wang, Z.-Q.; Xie, Y.-X.; Xia, Y.-Z.; Li, J.-H. J. Org. Chem. 2012, 77, 2837.
[19] (a) Orzeszko, B.; Melon-Ksyta, D.; Orzeszko, A. Synth. Commun. 2002, 32, 3425. (b) Kishikawa, K.; Inoue, T.; Sasaki, Y.; Aikyo, S.; Kohmoto, S.; Takahashi, M. Soft Matter. 2011, 7, 7532.
/
| 〈 |
|
〉 |