

负载型氮杂环卡宾钯络合物催化C—C偶联反应新进展
收稿日期: 2014-11-22
修回日期: 2014-12-21
网络出版日期: 2015-01-06
基金资助
江西省自然科学基金(No.20114BAB213011)、江西省教育厅基金(No.GJJ13447)、核资源与环境重点实验室(东华理工大学)开放基金(No.NRE1315)、东华理工大学博士科研启动基金(No.DHBK1010)、核资源与环境省部共建国家重点实验室培育基地(东华理工大学)开放基金(No.NRE1315)、国家自然科学基金(No.31101469)、新世纪优秀人才支持计划(No.NCET-12-0475)、陕西省青年科技新星(No.2012KJXX-16)、中央高校基本科研业务费(No.QN2011035)资助项目.
New Progress in C—C Coupling Reactions Catalyzed by Supported N-Heterocyclic Carbene Palladium Complex
Received date: 2014-11-22
Revised date: 2014-12-21
Online published: 2015-01-06
Supported by
Project supported by the Natural Science Foundation of Jiangxi Province (No.20114BAB213011), the Science and Technology Project of Jiangxi Provincial Department of Education (No.GJJ13447), the Open foundation of Key Laboratory of Nuclear Resources and Environment (East China Institute of Technology) (No.NRE1315), the Doctoral Scientific Research Foundation of East China of Technology (No.DHBK1010), the State Key Laboratory Breeding Base of Nuclear Resources and Environment (No.NRE1315), the National Natural Science Foundation of China (No.31101469), the New Century Excellent Talents by Ministry of Education of China (No.NCET-12-0475), the Fund of Youth Science and Technology Stars by Shaanxi Province (No.2012KJXX-16), and the Fundamental Research Funds for the Central Universities (No.QN2011035).
袁定重 , 张庆华 , 廖世军 , 熊文文 , 元利刚 , 蔡奇胜 , 杨梦梅 , 李雄 , 蒋烨佳 , 刘妍 , 李萍 , 徐贞帅 , 孙盼盼 , 耿会玲 . 负载型氮杂环卡宾钯络合物催化C—C偶联反应新进展[J]. 有机化学, 2015 , 35(5) : 961 -974 . DOI: 10.6023/cjoc201407022
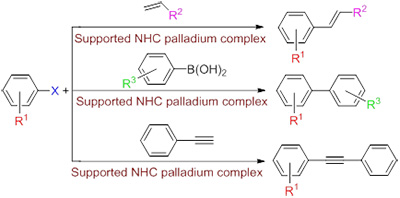
The C—C coupling reactions catalyzed by supported N-heterocyclic carbene palladium complex have attracted considerable attention because catalytic activity is excellent and supported catalyst can be separated easily and reused several times. The performance of the carriers has an important effect on the catalytic activity and reusability of the supported catalyst. In this paper, the advances in the C—C coupling reactions catalyzed by supported N-eterocyclic carbene palladium complex have been reviewed. Polymer, silica, carbon and magnetic nanoparticles were used to support the N-heterocyclic carbene palladium catalysts. Finally, the direction of the supported N-heterocyclic carbene palladium complex was also put forward.
[1] Molnar, A. Chem. Rev. 2011, 111, 2251.
[2] Heck, R. F. Org. React. 1982, 27, 345.
[3] Han, F. S. Chem. Soc. Rev. 2013, 42, 5270.
[4] Crisp, G. T. Chem. Soc. Rev. 1998, 27, 427.
[5] Cartney, D. M.; Guiry, P. Chem. Soc. Rev. 2011, 40, 5122.
[6] Kealey, S.; Huiban, M. Chem. Commun. 2013, 49, 11326.
[7] Liu, L. F.; Dong, Y.; Tang, N. N. Green Chem. 2014, 16, 2185.
[8] Fleckenstein, C. A.; Plenio, H. Green Chem. 2007, 9, 1287.
[9] Marziale, A. N.; Faul, S. H.; Reiner, T.; Schneider, S.; Eppinger, J. Green Chem. 2010, 12, 35.
[10] Tu, T.; Wang, Z. X.; Liu, X. Y. Dalton Trans. 2010, 39, 10598.
[11] Roy, S.; Plenio, H. Adv. Synth. Catal. 2010, 352, 1014.
[12] Li, L. Y.; Wang, J. Y.; Zhou, C. S.; Wang, R. H.; Hong, M. C. Green Chem. 2011, 13, 2100.
[13] Godoy, F.; Segarra, C.; Poyatos, M.; Peris, E. Organometallics 2011, 30, 684.
[14] Ranganath, K, V. S.; Onitsuka, S.; Kumar, A. K.; Inanaga, J. J. Catal. Sci. Technol. 2013, 3, 2161.
[15] Schwarz, J.; Bohm, V. P. W.; Gardiner, M. G.; Grosche, M.; Herrmann, W. A.; Hieringer, W.; Sieber, G. R. Chem. Eur. J. 2000, 6, 1773.
[16] Byun, J. W.; Lee. Y. S. Tetrahedron Lett. 2004, 45, 1837.
[17] Kim, J, H.; Jun, B. H.; Byun, J. W.; Lee, Y. S. Tetrahedron Lett. 2004, 45, 5827.
[18] Kim, J. H.; Kim, J. W.; Shokouhimehr, M.; Lee, Y. S. J. Org. Chem. 2005, 70, 6714.
[19] Shokouhimehr, M.; Kim, J. H.; Lee, Y. S. Synlett 2006, 618.
[20] Kim, J. H.; Lee, D. H.; Hyun, B.; Lee, Y. S. Tetrahedron Lett. 2007, 48, 7079.
[21] (a) Kang, T.; Feng, Q.; Luo, M. M. Synlett 2005, 2305. (b) Zhang, S.; Zeng, X.; Wei, Z.; Zhao, D.; Kang, T.; Zhang, W.; Yan, W.; Luo, M. M. Synlett 2006, 1891. (c) Qin, Y.; Wei, W.; Luo, M. Synlett 2007, 2410. (d) Nan, G.; Ren, F.; Luo, M. M. Beilstein J. Org. Chem. 2010, 6, 70.
[22] Zeng, X. M.; Zhang, T. X.; Qin, Y. C.; Wei, Z. J.; Luo, M. M. Dalton. Trans. 2009, 8341.
[23] Steel, P. G.; Teasdale, C. W. T. Tetrahedron Lett. 2004, 45, 8977.
[24] Altava, B.; Burguete, M. I.; Verdugo, E. G.; Karbass, N.; Luis, S. V.; Puzary, A.; Sans, V. Tetrahedron Lett. 2006, 47, 2311.
[25] Qureshi, Z. S.; Deshmukh, K. M.; Tambade, J.; Bhanage, B. M. Synthesis 2011, 243.
[26] Bergbreiter, D. E.; Su, H.-L.; Koizumi, H.; Tian, J. J. Or-ganomet. Chem. 2011, 696, 1272.
[27] Sommer, W. J.; Weck, M. Adv. Synth. Catal. 2006, 348, 2101.
[28] Lee, D. H.; Kim, J. H.; Hyun, B.; Kang, H.; Park, J.; Lee, Y. S. Org. Lett. 2008, 10, 1609.
[29] Yu, T.; Li, Y.; Yao, C. F.; Wu, H. H.; Liu, Y. M.; Wu, P. Chin. J. Catal. 2011, 32, 1712.
[30] Lin, M. J.; Wang, S. J.; Zhang, J. Y.; Luo, W. J.; Liu, H. L.; Wang, W.; Su, C.-Y. J. Mol. Catal. A: Chem. 2014, 394, 33.
[31] Xu, S, J.; Song, K. P.; Li, T.; Tan, B. E. J. Mater. Chem. A 2015, 3, 1272.
[32] (a) Liu, N.; Liu, C.; Jin, Z.-L. Chin. J. Org. Chem. 2012, 32, 860 (in Chinese). (刘宁, 刘春, 金子林, 有机化学, 2012, 32, 860.) (b) Park, G.; Lee, S.; Son, S. J.; Shi, S. Green Chem. 2014, 16, 2587. (c) Zhang, B. B.; Song, J. L.; Liu, H. Z.; Shi, J. H.; Ma, J.; Fan, H. L.; Wang, W. T.; Zhang, P.; Han, B. X. Green Chem. 2014, 16, 1198. (d) Ding, G. D.; Wang, W. T.; Jiang, T.; Han, B. X. Green Chem. 2013, 15, 3396.
[33] Meise, M.; Haag, R. ChemSusChem 2008, 1, 637.
[34] Liu, N.; Liu, C.; J. Z. L. Green Chem. 2012, 14, 592.
[35] Shi, J. C.; Yu, H. W.; Jiang, D. H.; Yu, M.; Huang, Y. X.; Nong, L. P.; Zhang, Q.; Jin, Z. L. Catal. Lett. 2014, 144, 158.
[36] Xue, J.; Zhou, Z. G.; Peng. J.; Du, F.; Xie, L. F.; Xu, G. H.; Huang, G. P.; Xie, Y. R. Transition Met. Chem. 2014, 39, 221.
[37] Williams, K. A.; Boydston, A. J.; Bielawski, C. W. Chem. Soc. Rev. 2007, 36, 729.
[38] (a) Karimi, B.; Akhavan, P. F. Chem. Commun. 2009, 3750. (b) Karimi, B; Akhavan, P. F. Chem. Commun. 2011, 47, 7686. (c) Karimi, B; Akhavan, P. F. Inorg. Chem. 2011, 50, 6063.
[39] Wang, X. X.; Hu, P. B.; Xue, F. J.; Wei, Y. P. Carbohydr. Polym. 2014, 114, 476.
[40] Aksin, O.; Tuerkmen, H.; Artok, L.; Cetinkaya, B.; Ni, C.; Bueyuekguengoer, O.; Oezkal, E. J. Organomet. Chem. 2006, 691, 3027.
[41] (a) Polshettiwar, V.; Hesemann, P.; Moreau, J. J. E. Tetrahedron Lett. 2007, 48, 5363. (b) Polshettiwar, V.; Varma, R. S. Tetrahedron 2008, 64, 4637.
[42] Lee, S. M.; Yoon, H. J.; Kim, J. H.; Chung, W. J.; Lee, Y. S. Pure Appl. Chem. 2007, 79, 1553.
[43] Ghiaci, M.; Zarghani, M.; Khojastehnezhad, A.; Moeinpour, F. RSC Adv. 2014, 4, 15496.
[44] Martinez, A.; Krinsky, J. Penafiel, I.; Castillon, S.; Loponov, K.; Lapkin, A.; Godard, C.; Claver, C. Catal. Sci. Technol. 2015, 5, 310.
[45] Yang, H. Q.; Li, G. A.; Ma, Z. C.; Chao, J. B.; Guo, Z. Q. J. Catal. 2010, 276, 123.
[46] Yang, H. Q.; Han, X. J.; Li, G.; Wang, Y. W. Green Chem. 2009, 11, 1184.
[47] Borja, G.; Monge-Marcet, A.; Pleixats, R.; Parella, T.; Cattoen, X.; Man, M. W. C. Eur. J. Org. Chem. 2012, 3625.
[48] Scheuermann, G. M.; Rumi, L.; Steurer, P. J. Am. Chem. Soc. 2009, 131, 8262.
[49] Chua, C. K.; Pumera, M. Chem. Soc. Rev. 2014, 43, 291.
[50] Upadhyay, R. K.; Soin, N.; Roy, S. S. RSC Adv. 2014, 4, 3823.
[51] Li, G.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. J. Mater. Chem. A 2014, 2, 9185.
[52] Siamaki, A. R.; Khder, A. E. R. S.; Abdelsayed, V.; El-Shall, M. S.; Gupton, B. F. J. Catal. 2011, 279, 1.
[53] Shang, N. Z.; Gao, S.; Feng, C. Zhang, H. Y.; Wang, C.; Wang, Z. RSC Adv. 2013, 3, 21863.
[54] Movahed, K. S.; Esmatpoursalmani, R.; Bazgir, A. RSC Adv. 2014, 4, 14586.
[55] Park, J. H.; Raza, F.; Jeon, S.-J.; Kim, H.-I.; Kang, T. W.; Yim, D. B.; Kim, J.-H. Tetrahedron Lett. 2014, 55, 3426.
[56] (a) Yuan, D.-Z.; Huang, B. Chin. J. Org. Chem. 2012, 32, 1368 (in Chinese). (袁定重, 黄斌, 有机化学, 2012, 32, 1368.) (b) Yuan, D. Z.; Zhang, H. P. J. Appl. Catal. A: Gen. 2014, 475, 249. (c) Yuan, D. Z.; Zhang, Q. Y.; Dou, J. B. Catal. Commun. 2010, 11, 606. (d) Yuan, D. Z.; Zhang, Q. Y.; Dou, J. B. Chin. Chem. Lett. 2010, 21, 1062.
[57] Gawande, M. B.; Brancoa, P. S.; Varma, R. S. RSC Adv. 2013, 42, 3371.
[58] Shylesh, S.; Schunemann, V.; Thiel, W. R. Angew. Chem., Int. Ed. 2010, 49, 3428.
[59] Wu, Z. W.; Li, Z.; Wu, G. M.; Wang, L.; Lu, S. Q.; Wang, L.; Wan, H.; Guan, G. F. Ind. Eng. Chem. Res. 2014, 53, 3040.
[60] Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H. B.; Bouhrara, M.; Basset, J. M. Chem. Rev. 2011, 111, 3036.
[61] Stevens, P. D.; Li, G.; Fan, J.; Yen, M.; Gao, Y. Chem. Commun. 2005, 4435.
[62] Zheng, Y.; Stevens, P. D.; Gao, Y. J. Org. Chem. 2006, 71, 537.
[63] Lu, A. H.; Salabas, E. L.; Schuth, F. Angew. Chem., Int. Ed. 2007, 46, 1222.
[64] Yang, H. Q.; Wang, Y. W.; Qin, Y.; Chong, Y. Z.; Yang, Q. Z.; Li, G.; Zhang, L.; Li, W. Green Chem. 2011, 13, 1352.
[65] Ghotbinejad, M.; Khosropour, A. R.; Baltork, I. M.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. J. Mol. Catal. A: Chem. 2014, 385, 78.
[66] Stevens, P. D.; Fan, J.; Gardimalla, H. M. R.; Yen, M.; Gao, Y. Org. Lett. 2005, 7, 2085.
[67] Wang, Z.; Yu, Y.; Zhang, Y. X.; Li, S. Z.; Qian, H.; Lin, Z. Y. Green Chem. 2015, 17, 413.
/
| 〈 |
|
〉 |