

新型取代3-[(5-苄硫-1,3,4-噁二唑-2-基)甲基]苯并[d]噻(噁)唑-2(3H)-酮的合成及杀菌活性
收稿日期: 2014-10-31
修回日期: 2014-12-14
网络出版日期: 2015-01-07
基金资助
国家自然科学基金(No.30900959)和浙江省公益性(No.2014C31127)资助项目.
Synthesis and Antifungal Activity of Novel 3-[(5-Benzylthio- 1,3,4-oxadiazol-2-yl)methyl]benzo[d]thiazol- (oxazol)-2(3H)-ones
Received date: 2014-10-31
Revised date: 2014-12-14
Online published: 2015-01-07
Supported by
Project supported by the National Natural Science Foundation of China (No.30900959) and Public Project of Zhejiang Province (No.2014C31127).
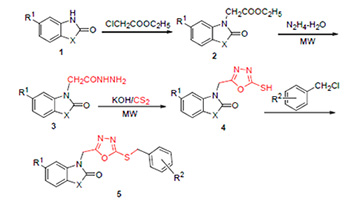
为寻找新型高效杂环农药先导化合物, 以取代苯并噻(噁)唑酮为原料, 经取代、肼化、环化、苄基化反应合成了21个新型取代3-[(5-苄硫-1,3,4-噁二唑-2-基)甲基]苯并[d]噻(噁)唑-2(3H)-酮类化合物, 并利用1H NMR, IR, EI-MS及元素分析对其结构进行表征. 初步生物活性试验结果表明, 在50 mg/L浓度下, 大部分化合物对黄瓜炭疽病菌(Colletotrichum orbiculare), 灰葡萄孢菌(Botrytis cinerea)和水稻纹枯病菌(Rhizoctonia solani)具有中等杀菌活性, 其中化合物5b对灰葡萄胞菌和水稻纹枯病菌的抑制率均达到了85%以上.
阮铃莉 , 范人杰 , 刘幸海 , 陈杰 , 翁建全 . 新型取代3-[(5-苄硫-1,3,4-噁二唑-2-基)甲基]苯并[d]噻(噁)唑-2(3H)-酮的合成及杀菌活性[J]. 有机化学, 2015 , 35(5) : 1166 -1172 . DOI: 10.6023/cjoc201410041
In order to find novel biologically active pesticide lead compounds, twenty-one novel 3-[(5-benzylthio-1,3,4- oxadiazol-2-yl)methyl]benzo[d]thiazol(oxazol)-2(3H)-ones were synthesized by 2-benzothiazolinone/benzoxazolone as starting materials via substitution, hydrazine, cyclization and the last benzylation reaction. The structures of the title compounds were characterized by 1H NMR, IR, EI-MS and elemental analysis. The preliminary bioassay results indicated that most of them showed moderate inhibition activity against Colletotrichum orbiculare, Botrytis cinerea and Rhizoctonia solani at 50 mg/L, and the inhibition rate of compound 5b against Botrytis cinerea and Rhizoctonia solani reached above 85%.

[1] Strange, R. N.; Scott, P. R. Annu. Rev. Phytopathol. 2005, 43, 83.
[2] Li, B.; Liu, B.-P.; Yu, R.-R.; Lou, M.-M.; Wang, Y.-L.; Xie, G.-L.; Li, H.-Y.; Sun, G.-C. World J. Microbiol. Biotechnol. 2011, 27, 2305.
[3] Groth, D. E. Crop Prot. 2008, 27, 1125.
[4] Mahran, M. A.; Einassry, S. M.; Allam, S. R. Pharmazie 2003, 58, 527.
[5] Sidoova, E.; Loos, D.; Bujdakova, H. Molecules 1997, 2, 36.
[6] Wang, W.; Zhang, G.-P.; Song, B.-A.; Wang, H.; Jin, L.-H.; Hu, D.-Y.; Yang, S. Chin. J. Org. Chem. 2007, 27, 279 (in Chinese). (王伟, 张国平, 宋宝安, 汪华, 金林红, 胡德禹, 杨松, 有机化学, 2007, 27, 279.)
[7] Ganzer, M.; Dorfmeister, G.; Franke, W.; Johann, G.; Rees, R. DE 4117508, 1992 [Chem. Abstr. 1993, 118, 101946].
[8] David, P. C.; Roy, V. E.; Roy, T. H. J. Agric. Food Chem. 1981, 29, 640.
[9] Gewehr, M.; Dietz, J.; Grote, T.; Haden, E. WO 2009147205, 2009 [Chem. Abstr. 2010, 152, 113128].
[10] Michel, S. US 3922281, 1975 [Chem. Abstr. 1975, 82, 170877].
[11] Toyabe, K.; Shimizu, K.; Hirata, M.; Ikeda, T. JP 0217187, 1990 [Chem. Abstr. 1990, 113, 59158].
[12] Kraus, A.; Ishikawa, K. WO 2004095930, 2004 [Chem. Abstr. 2004, 141, 390414].
[13] Sidoova, E.; Loos, D.; Bujdakova, H.; Kallova, J. Molecules 1997, 2, 36.
[14] Koci, J.; Klimesova, V.; Waisser, K. Bioorg. Med. Chem. Lett. 2002, 12, 22.
[15] Hutchinson, I.; Chua, M. S.; Browne, H. L. J. Med. Chem. 2001, 4, 9.
[16] Huang, Q.-C.; Qian, X.-H.; Song, G.-H.; Cao, S. Pest Manage. Sci. 2003, 59, 933.
[17] Mo, Q.-J.; Duan, W.-G.; Li, X.-R.; Huang, D.-P.; Luo, D.-C. Chin. J. Org. Chem. 2011, 31, 1114 (in Chinese). (莫启进, 段文贵, 李行任, 黄丹平, 罗德城, 有机化学, 2011, 31, 1114.)
[18] Omar, F.-A.; Mahfouz, N.-M.; Rahman, M.-A. Eur. J. Med. Chem. 1996, 31, 819.
[19] Li, C.; Tan, Z.-L.; Li, X.-W.; Zhang, X. Chin. J. Org. Chem. 2005, 25, 5 (in Chinese). (李超, 覃章兰, 李秀文, 张欣, 有机化学, 2005, 25, 5.)
[20] Li, Q.-Z.; Song, B.-A.; Chen, J. Agrochemicals 2005, 44, 12 (in Chinese). (李黔柱, 宋宝安, 陈江, 农药, 2005, 44, 12.)
[21] Weng, J.-Q.; Liu, X.-H.; Huang, H.; Tan, C.-X.; Chen, J. Molecules 2012, 17, 989.
[22] Che, C.; Mao, S.-F.; Tan, Z.-H. Chin. Appl. Chem. 2002, 19, 8 (in Chinese). (车超, 毛淑芬, 覃兆海, 应用化学, 2002, 19, 8.)
[23] Elwahy, A. H.; Abbas, A. A. Tetrahedron 2000, 56, 885.
[24] Chen, H.-S.; Li, Z.-M.; Han, Y.- F. J. Agric. Food Chem. 2000, 48, 5312.
[25] Bao, X.-P.; Lin, X.-F.; Zhang, F.; Zhou, L.-B. Chin. J. Org. Chem. 2013, 33, 995 (in Chinese). (鲍小平, 林选福, 张峰, 邹林波, 有机化学, 2013, 33, 995.)
[26] Zhong, B.; Fan, Z.-J.; Li, Z.-M. Chin. Appl. Chem. 2003, 20, 7 (in Chinese). (钟斌, 范志金, 李正名, 应用化学, 2003, 20, 7.)
[27] De Sousa, G.; Nawaz, A.; Cravedi, J. P.; Rahmani, R. Toxicol. Sci. 2014, 141, 1.
[28] Chen, W.-B.; Jin, G.-Y. Acta Chim. Sinica 2001, 22, 1147 (in Chinese). (陈文彬, 金桂玉, 化学学报, 2001, 22, 1147.)
[29] Wang, L.; Asimakopoulos, A. G.; Moon, H. B.; Nakata, H.; Kannan, K. Environ. Sci. Technol. 2013, 47, 9.
[30] Amico, D. J.; Bollinger, F. G. Heterocycl. Chem. 1988, 25, 1183.
[31] Li, Y.-J.; Li, C.-Y.; Sun, S.-Q.; Zhou, X.-X. Acta Chim. Sinica 2012, 70, 2 (in Chinese). (李英俊, 李春燕, 孙淑琴, 周晓霞, 化学学报, 2012, 70, 2.)
/
| 〈 |
|
〉 |