

高异黄酮类化合物的合成及其舒张血管活性研究
收稿日期: 2014-11-20
修回日期: 2014-12-30
网络出版日期: 2015-01-14
基金资助
陕西省科技统筹创新工程项目基金(No.2012KPCL03-20)资助项目.
Synthesis and Vasodilatation of Homoisoflavones
Received date: 2014-11-20
Revised date: 2014-12-30
Online published: 2015-01-14
Supported by
Project supported by the Innovation Project on Science and Technology of Shanxi Province (No.2012KPCL03-20).
郑国勋 , 张宗昌 , 康博瑞 , 于瑞红 , 曹永孝 , 张三奇 . 高异黄酮类化合物的合成及其舒张血管活性研究[J]. 有机化学, 2015 , 35(5) : 1112 -1122 . DOI: 10.6023/cjoc201411033
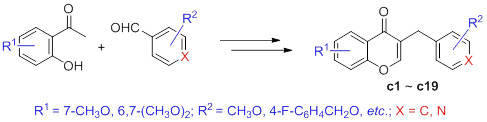
Homoisoflavones were designed as vasodilative agents on the basis of scaffold hopping. A series of homoisoflavones were synthesized from 2-acetylphenol and aromatic aldehyde via aldol condensation, hydrogenation and cyclization. The structures of title compounds were characterized by 1H NMR, MS and HRMS. The vasodilative effects of synthesized compounds were evaluated by wire myograph on isolated rat mesenteric arterial ring induced contraction with 60 mmol/L KCl. The results suggest that the title compounds are novel vasodilative agents.
Key words: homoisoflavones; synthesis; vasodilatation
[1] (a) Jiang, H. B.; Huang, J.; Guo, M. J.; Zou, P.; Tian, X. Q. Acta Pharm. Sin. 2007, 42, 118 (in Chinese). (江洪波, 黄静, 郭明娟, 邹萍, 田祥琴, 药学学报, 2007, 42, 118.) (b) Gaspar, A.; Matos, M. J.; Garrido, J.; Uriarte, E.; Borges, F. Chem. Rev. 2014, 114, 4960. (c) Keri, R. S.; Budagumpi, S.; Pai, R. K.; Balakrishna, R. G. Eur. J. Med. Chem. 2014, 78, 340.
[2] Min, B. S.; Cuong, T. D. Nat. Prod. Sci. 2013, 19, 201.
[3] Li, N.; Zhang, J. Y.; Zeng, K. W.; Zhang, L.; Che, Y. Y.; Tu, P. F. Fitoterapia 2012, 83, 1042.
[4] Rao, V. M.; Damu, G. L. V.; Sudhakar, D.; Siddaiah, V.; Rao, C. V. ARKIVOC 2008, 11, 285.
[5] Siddaiah, V.; Rao, C. V.; Venkateswarlu, S.; Subbaraju, G. V. Tetrahedron 2006, 62, 841.
[6] Lin, L. G.; Xie, H.; Li, H. L.; Tong, L. J.; Tang, C. P.; Ke, C. Q.; Liu, Q. F.; Lin, L. P.; Geng, M. Y.; Jiang, H. L.; Zhao, W. M.; Ding, J.; Ye, Y. J. Med. Chem. 2008, 51, 4419.
[7] Billah, M. M.; Islam, R.; Khatun, H.; Parvin, S.; Islam, E.; Islam, S. A.; Mia, A. A. BMC Complementary Altern. Med. 2013, 13, 101.
[8] Shaikh, M. M.; Kruger, H. G.; Smith, P.; Munro, O. Q.; Bodenstein, J.; Toit, K. D. J. Pharm. Res. 2013, 6, 1.
[9] Zhang, S. Q.; Li, Q.; Zhu, Y. L.; Cao, Y. X.; Liu, R. X.; Chen, Z. G. Chin. J. Org. Chem. 2009, 29, 966 (in Chinese). (张三奇, 李强, 祝丽永, 曹永孝, 刘瑞熙, 陈战国, 有机化学, 2009, 29, 966.)
[10] Zuo, S. J.; Li S.; Yu, R. H.; Zheng, G. X.; Cao, Y. X.; Zhang, S. Q. Bioorg. Med. Chem. Lett. 2014, 24, 5597.
[11] Wu, J. H.; Li, Q.; Wu, M. Y.; Guo, D. J.; Chen, H. L.; Chen, S. L.; Seto, S. W.; Au, A. L.; Poon, C. C.; Leung, G. P.; Lee, S. M.; Kwan, Y. W.; Chan, S. W. J. Nutr. Biochem. 2010, 21, 613.
[12] Sun, T.; Wang, J.; Huang, L. H.; Cao, Y. X. Eur. J. Pharm. 2013, 699, 241.
[13] Jaspal, S. J. Pharm. Res. 2012, 5, 439.
[14] Takeshi, K.; Ushio, S.; Tomoko, T.; Kenichi, A.; Nobutaka, T. Chem. Pharm. Bull. 1985, 33, 4109.
[15] Liu, X. F.; Shi, D. H. Appl. Chem. Ind. 2009, 38, 1210 (in Chinese). (刘翔峰, 史道华, 应用化工, 2009, 38, 1210.)
[16] Guan, L. P.; Yin, X. P.; Quan, H. M.; Quan, Z. S. Chin. J. Org. Chem. 2004, 24, 1274 (in Chinese). (关丽萍, 尹秀梅, 全红梅, 全哲山, 有机化学, 2004, 24, 1274.)
[17] Yang, J. H.; Meng, L. C. Chin. J. Synth. Chem. 2007, 15, 740 (in Chinese). (杨金会, 孟丽聪, 合成化学, 2007, 15, 740.)
[18] Dang, S.; Liu, J. G.; Wang, G. H. Chin. J. Synth. Chem. 2008, 16, 460 (in Chinese). (党珊, 刘锦贵, 王国辉, 合成化学, 2008, 16, 460.)
[19] Yu, H. T.; Kang, R. H.; Ouyang, X. M. Chin. J. Org. Chem. 2000, 20, 441 (in Chinese). (于海涛, 康汝洪, 欧阳兴梅, 有机化学, 2000, 20, 441.)
[20] Kirkiacharian, B. S.; Gomis, M. Synth. Commun. 2005, 35, 563.
[21] Fang,. X. B.; Gao, Y.; Nie, G. H.; Dai, K. Fine Chem. Inter. 2012, 42, 46 (in Chinese). (方旭兵, 高莹, 聂光辉, 温超, 戴康, 精细化工中间体, 2012, 42, 46.)
/
| 〈 |
|
〉 |