

132-氧代焦脱镁叶绿酸的化学反应及其叶绿素类二氢卟吩衍生物的合成
收稿日期: 2014-06-28
修回日期: 2014-09-09
网络出版日期: 2015-01-28
基金资助
国家自然科学基金(No. 21272048)和山东省黄金工程技术研究中心(2011年度)资助项目.
Chemical Reactions of 132-Oxo-pyropheophorbide and Synthesis of Chlorophyllous Chlorins Derivatives
Received date: 2014-06-28
Revised date: 2014-09-09
Online published: 2015-01-28
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272048) and the Project of Shandong Applied Reaearch Centre of Gold Nanotechnology (2011).
以焦脱镁叶绿酸-a甲酯及其12-氧代衍生物为起始原料, 利用试剂氧化和空气氧化在周环上构建了不同的含氧官能团, 再通过E-环羰基的Wittig和Grignard反应、20-meso-位的亲电取代反应和3-位乙烯基的1,3-偶极环加成等经典反应进行官能团转换, 讨论了132-氧代焦脱镁叶绿酸的分子结构、反应位点及其化学选择性, 完成了一系列未见报道的叶绿素类二氢卟吩衍生物的合成, 其化学结构均经UV、IR、1H NMR及元素分析予以证实.
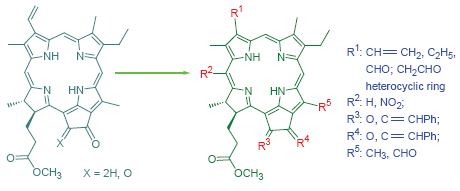
关键词: 叶绿素-a; 二氢卟吩; 132-氧代焦脱镁叶绿酸; 化学反应; 合成
杨晓英 , 张善国 , 张朋 , 张千 , 王振 , 金英学 , 祁彩霞 , 王进军 . 132-氧代焦脱镁叶绿酸的化学反应及其叶绿素类二氢卟吩衍生物的合成[J]. 有机化学, 2015 , 35(1) : 181 -190 . DOI: 10.6023/cjoc201406047
Pyropheophorbide-a methyl ester and its 132-oxoed derivatives were used as starting material, and the oxygen-containing functional groups were established on the chlorin periphery by oxygenation with agent and allomerization. The functional group transformation was carried out by classic reactions including Wittig and Grignard reactions in E-ring, electrophilic substitution at 20-meso-position and 1,3-dipolar cyclocaddition with C(3)-vinyl group. The molecule structure, reactive site and chemical selectivity of 132-oxopyropheophorbide-a were also discussed. The syntheses of a series of unreported chlorins related to chlorophyll were accomplished and their chemical structures were characterized by elemental analysis, UV, IR and 1H NMR spectra.

Key words: chlorophyll-a; chlorin; 132-oxopyropheophorbide-a; chemical reaction; synthesis
[1] Agius, L.; Ballantine, J. A.; Ferrito, V.; Jaccarini, V.; Murray, J. P.; Peter, A. F.; Schembri, P. J. Pure Appl. Chem. 1973, 51, 1847.
[2] (a)Kozyrev, A. N.; Alderfer, F. L.; Srikrishnan, T.; Pandeyk, R. K. J. Chem. Soc., Perkin Trans. 1 1998, 837. (b) Chen, Y. H.; Li, G. L.; Pandey, R. K. Curr. Org. Chem. 2004, 8, 1105. (c) Wang, J.-J. Chin. J. Org. Chem. 2005, 25, 1353 (in Chinese). (王进军, 有机化学, 2005, 25, 1353.) (d) Yang, Z.; Wang, Z.; Liu, Y.; Xu, X.-S.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2013, 33, 116 (in Chinese). (杨泽, 王振, 刘洋, 徐希森, 祁彩霞, 王进军, 有机化学, 2013, 33, 116.)
[3] (a) Kozyrev, A.; Ethirajan, M.; Chen, P.; Ohkubo, K.; Robinson, B. C.; Berkigia, K. M.; Fukuzumi, S.; Kadish, K. M.; Pandey, R. K. J. Org. Chem. 2012, 77, 10260. (b) Pavlov, V. Y.; Ponomarev, G. V. Chem. Heterocycl. Compd. 2004, 40, 393. (c) Kozyrey, A. N.; Chen, Y.-H.; Goswami, L. N.; Tabaczynaki, W. A.; Pandey, R. K. J. Org. Chem. 2006, 71, 1949. (d) Liu, R.-R.; Wang, L.-M.; Yin, J.-G.; Wu, J.; Liu, C.; Zhang, P.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 318 (in Chinese). (刘冉冉, 王鲁敏, 金英学, 武进, 刘超, 王朋, 王进军, 有机化学, 2012, 32, 318.)
[4] (a) Wang, J.-J.; Yin, Y.-F.; Yang, Z. J. Iran. Chem. Soc. 2013, 10, 583. (b) Wang, L.-M.; Wang, P.; Liu, C.; Jin, Y.-X.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 1700 (in Chinese). (王鲁敏, 王朋, 刘超, 金英学, 王进军, 有机化学, 2012, 32, 1700.) (c) Wang, J.-J.; Wang, P .; Li, J.-Z.; Jakus, J.; Shin, Y.-K. Bull. Korean Chem. Soc. 2011, 32, 3473. (d) Li, J.-Z.; Wang, J.-J.; Yoon, L.; Cui, B.-C.; Shim, Y.-K. Bioorg. Med. Chem. Lett. 2012, 22, 1846.
[5] (a) Li, J.-Z.; Zhang, P.; Yao, N.-N.; Zhao, L.-L.; Wang, J.-J.; Shim, Y.-K. Tetrahedron Lett. 2014, 55, 1086. (b) Li, J.-Z.; Liu, W.-H.; Li, F.-G.; Wang, J.-J.; Suo, Y.-R.; Liu, Y.-J. Chin. J. Org. Chem. 2007, 27, 1594 (in Chinese). (李家柱, 刘万卉, 李付国, 王进军, 索有瑞, 刘永军, 有机化学, 2007, 27, 1594.) (c) Wang, J.-J.; Li, J.-Z.; Li, Y.-W.; Jakus, J.; Shim, Y.-K. J. Porphyrins Phthalocyanines 2010, 14, 859
[6] (a) Yu, S.-S.; X, X.-S.; Liu, Y.; Li, J.-Z.; Jin, Y.-X.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2014, 34, 362 (in Chinese). (于沙沙, 徐希森, 刘洋, 李家柱, 金英学, 祁彩霞, 王进军, 有机化学, 2014, 34, 362.) (b) Tamiaki, H.; Monobe, R.; Koizumi, S.; Miyatake, T.; Kinoshita, Y. Tetrahedron: Asymmerty 2013, 24, 966. (c) Srivatsan, A.; Wang, Y.-F.; Joshi, P.; Sajjad, M.; Chen, Y.-H.; Liu, C.; Thankcppan, K.; Missert, J. R.; Tracy, E.; Morgan, J.; Rigual, N.; Baumann, H.; Pandey, R. K. J. Med. Chem. 2011, 54, 6859.
[7] Ji, J.-Y.; Wang, L.M.; Jing, J.-R.; Han, G.-F.; Wang, J. J. Chin. J. Org. Chem. 2007, 27, 493 (in Chinese). (纪建业, 王鲁民, 荆济荣, 韩光范, 王进军, 有机化学, 2007, 27, 493.)
[8] Liu, C. M.S. Thesis, Yantai University, Yantai, 2010 (in Chinese). (刘超, 硕士论文, 烟台大学, 烟台, 2010.)
[9] (a) Yin, J.-G.; Wang, Z.; Yang, Z.; Liu, C.; Zhao, L.-L.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 360 (in Chinese). (殷军港, 王振, 杨泽, 刘超, 赵丽丽, 王进军, 有机化学, 2012, 32, 360.) (b) Wang, J.-J.; Zhao, Y.; Wu, X.-R.; Han, G.-F.; Shin, R.-K. Chin. J. Org. Chem. 2002, 22, 565 (in Chinese). (王进军, 赵岩, 邬旭然, 韩光范. 沈荣基, 有机化学, 2002, 22, 565.)
[10] Liu, R. R. M.S. Thesis, Yantai University, Yantai, 2011 (in Chinese). (刘冉冉, 硕士论文, 烟台大学, 烟台, 2011.)
[11] Wang, L.-M.; Wang, P.; Liu, C; Jin, Y.-X.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 1707 (in Chinese). (王鲁敏, 王朋, 刘超, 金英学, 王进军, 有机化学, 2012, 32, 1707.)
[12] Liu, Y. M.S. Thesis, Yantai University, Yantai, 2014 (in Chinese). (刘洋, 硕士论文, 烟台大学, 烟台, 2014.)
[13] Tamiaki, H.; Miyatake, T.; Tanikaga, R. Tetrahedron Lett. 1997, 38, 267.
[14] Smith, K. M.; Gogg, D. A.; Simpson, D. J. J. Am. Chem. Soc. 1985, 107, 4946.
/
| 〈 |
|
〉 |