

二芳基苯并1,3-噻嗪烷-4-酮衍生物的微波促进合成及其HIV逆转录酶抑制活性
收稿日期: 2014-12-30
修回日期: 2015-02-03
网络出版日期: 2015-02-05
基金资助
国家自然科学基金(No. 21372060)、河北省自然科学基金石药集团医药联合基金(No. B2012201113)、河北省教育厅自然科学基金(No. Y2011119)、保定市科技局(No. 14ZF095)资助项目.
Microwave Assisted Synthesis and Anti-HIV-RT Activity of Diaryl Benzo[1,3]thiazin-4-ones
Received date: 2014-12-30
Revised date: 2015-02-03
Online published: 2015-02-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372060), the Medicinal Joint Funds of the Natural Science Foundation of Hebei and Shijiazhuang Pharmaceutical Group (No. B2012201113), the Natural Science Foundations of Education Department of Hebei (No. Y2011119), and the Foundations of Baoding City Science and Technology Bureau (No. 14ZF095).
以嘧啶胺、芳醛和硫代水杨酸为原料, 利用三组分一锅法, 微波辐射10 min, 合成了系列含新型二芳基苯并噻嗪烷-4-酮衍生物, 反应具有时间短、操作简便等优点. 目标化合物结构通过IR、NMR、MS及元素分析等确定. 2-(2,6-二氯苯基)-3-(4,6-二甲基嘧啶-2-基)-2H-1,3-苯并噻嗪烷-4-酮(10c)表现出良好的HIV-RT抑制活性, IC50值为8.38 μmol· L-1, 其余化合物则具有中等的酶抑制活性.
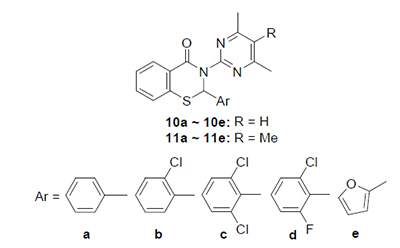
关键词: 苯并噻嗪烷-4-酮; HIV逆转录酶抑制活性; 微波辐射
冯俊娜 , 李晓慧 , 邵洁 , 朱墨 , 李妍 , 陈华 , 李小六 . 二芳基苯并1,3-噻嗪烷-4-酮衍生物的微波促进合成及其HIV逆转录酶抑制活性[J]. 有机化学, 2015 , 35(6) : 1370 -1374 . DOI: 10.6023/cjoc201412053
A series of novel diaryl benzothiazin-4-ones were designed and synthesized by the three-component one-pot cyclocondensation of pyrimidinamine, aromatic aldehyde and thiosalicylic acid under microwave irradiation for 10 min. The reaction has the advantages such as short reaction time and simple operation. The structures of the newly synthesized compounds were confirmed by IR, NMR, MS spectra and elemental analysis. The compound 2-(2,6-dichlorophenyl)-3-(4,6-di- methylpyrimidin-2-yl)-2H-benzo[e][1,3]thiazin-4(3H)-one (10c) could effectively inhibit HIV-RT activity with the IC50 value of 8.38 祄ol·L-1, the others showed middle RT inhibitory activity.

Key words: benzothiazin-4-one; anti-HIV-RT activity; microwave irradiation
[1] Mehellou, Y.; De Clercq, E. J. Med. Chem. 2010, 53, 521.
[2] Reynold, C.; de Koning, C. B.; Pelly, S. C.; van Otterlo, W. A. L.; Bode, M. L. Chem. Soc. Rev. 2012, 41, 4657.
[3] Zhan, P.; Chen, X. W.; Li, D. Y.; Fang, Z. J.; De Clercq, E.; Liu, X. Y. Med. Res. Rev. 2013, 33(suppl. 1), E1.
[4] Prajapati, D. G.; Ramajayam, R.; Yadav, M. R.; Giridhar, R. Bioorg. Med. Chem. 2009, 17, 5744.
[5] Verma, A.; Saraf, S. K. Eur. J. Med. Chem. 2008, 43, 897.
[6] Tian, Y.; Zhan, P.; Rai, D.; Zhang, J. Y.; De Clercq, E.; Liu, X. Y. Curr. Med. Chem. 2012, 19, 2026.
[7] Chen, H.; Bai, J.; Jiao, L. L.; Guo, Z. H.; Yin, Q. M.; Li, X. L. Bioorg. Med. Chem. 2009, 17, 3980.
[8] Ravichandran, V.; Prashantha Kumar, B. R.; Sankar, S.; Agrawal, R. K. Eur. J. Med. Chem. 2009, 44, 1180.
[9] Murugesan, V.; Prabhakar, Y. S.; Katti, S. B. J. Mol. Graphics Modell. 2009, 27, 735.
[10] Johannes, K.; Martens, J. Tetrahedron 2010, 66, 242.
[11] Zarghi, A.; Zebardast, T.; Daraie, B.; Hedayati, M. Bioorg. Med. Chem. 2009, 17, 5369.
[12] Geng, J. H.; Li, Y. X.; Zhang, W. J.; Su, X.; Guo, C. J. Shenyang Pharm. Univ. 2012, 29, 834 (in Chinese). (耿红健, 高宁, 李裕鑫, 张卫军, 苏昕, 郭春, 沈阳药科大学学报, 2012, 29, 834.)
[13] Tiwari, R.; Moraski, G. C.; Krchň?k, V.; Miller, P. A.; Colon-Martinez, M.; Herrero, E.; Oliver, A. G.; Miller, M. J. J. Am. Chem. Soc. 2013, 135, 3539.
[14] Makarov, V.; Manina, G.; Mikusova, K.; Möllmann, U.; Ryabova, O.; Saint-Joanis, B.; Dhar, N.; Pasca, M. R.; Buroni, S.; Lucarelli, A. P.; Milano, A.; De Rossi, E.; Belanova, M.; Bobovska, A.; Dianiskova, P.; Kordulakova, J.; Sala, C.; Fullam, E.; Schneider, P.; McKinney, J. D.; Brodin, P.; Christophe, T.; Waddell, S.; Butcher, P.; Albrethsen. J.; Rosenkrands, I.; Brosch, R.; Nandi, V.; Bharath, S.; Gaonkar, S.; Shandil, R. K.; Balasubramanian, V.; Balganesh, T.; Tyagi, S.; Grosset, J.; Riccardi, G.; Cole, S. T. Science 2009, 324, 801.
[15] Cao, X.; Wang, S. B.; Deng, X. Q.; Liu, D. C.; Quan, Z. S. Med. Chem. Res. 2014, 23, 1829.
[16] Stössel, A.; Schlenk, M.; Hinz, S.; Küppers, P.; Heer, J.; Gütschow, M.; Müller, C. E. J. Med. Chem. 2013, 56, 4580.
[17] Rawal, R. K.; Solomon, V. R.; Prabhakar, Y. S.; Katti, S. B.; De Clercq, E. Comb. Chem. High Throughput Screening 2005, 8, 439.
[18] Zhu, Y. J.; Chen, F. Chem. Rev. 2014, 114, 6462.
[19] Chen, H.; Bai, J.; Zhao, L.; Yuan, X. G.; Li, X. L.; Cao, K. Q. Chin. J. Org. Chem. 2008, 28, 1092 (in Chinese). (陈华, 白洁, 赵莲, 苑香果, 李小六, 曹克强, 有机化学, 2008, 28, 1092.)
[20] Zhou, Z. Z.; Huang, W.; Ji, F. Q.; Ding, M. W.; Yang, G. F. Heteroat. Chem. 2007, 18, 381.
[21] Chen, H.; Huang, C, J.; Zhu, M.; Li, X. L. Chin. J. Org. Chem. 2014, 34, 756 (in Chinese). (陈华, 黄长军, 朱墨, 李小六, 有机化学, 2014, 34, 756.)
[22] Reverse Transcriptase Assay, Colorimetric kit, Roche Diagnostics GmbH, Roche Applide Science, Sandhofer Strasse 116, D-68305 Mannheim, Germany.
/
| 〈 |
|
〉 |