

多组分反应在合成多取代咪唑类化合物中的应用
收稿日期: 2014-12-05
修回日期: 2015-02-09
网络出版日期: 2015-02-11
基金资助
河北省自然科学基金(No. B2013408014)和廊坊师范学院重点(No. LSLZ201402)资助项目.
Application of Multi-component Reaction in Synthesis of Multi- substituted Imidazoles
Received date: 2014-12-05
Revised date: 2015-02-09
Online published: 2015-02-11
Supported by
Project supported by the Natural Science Foundation of Hebei Province (No. B2013408014) and the Key Foundation of Langfang Teachers University (No. LSLZ201402).
肖立伟 , 彭晓霞 , 周秋香 , 寇伟 , 时亚茹 . 多组分反应在合成多取代咪唑类化合物中的应用[J]. 有机化学, 2015 , 35(6) : 1204 -1215 . DOI: 10.6023/cjoc201412012
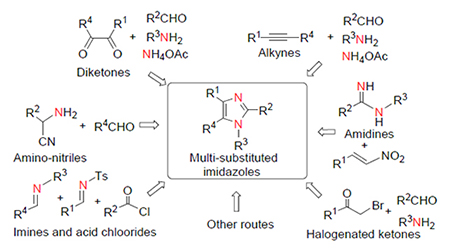
Multi substituted imidazoles including tri- and tetra-substituted imidazoles, play important roles in the field of pharmaceutical, chemical sensing and catalysis etc. Multi-component reaction (MCR), a protocol with high atom economic and reaction selectivity, has been applied widly on the synthesis of multi-substituted imidazoles. Based on different starting materials and different methods, the recent advances in the synthesis of multi-substituted imidazols via multi-component reaction are reviewed.
[1] Koch, P.; Bauerlein, C.; Jank, H.; Laufer, S. J. Med. Chem. 2008, 51, 5630.
[2] Li, W. J.; Li, Q.; Liu, D. L.; Ding, M. W. J. Agric. Food Chem. 2013, 61, 1419.
[3] Xu, Z. X.; Wang, S. M.; Zhao, L.; Zhang, S. L.; Li, J. B. Chin. J. Org. Chem. 2003, 23(9), 950 (in Chinese). (许祖勋, 王世敏, 赵雷, 张胜利, 黎俊波, 有机化学, 2003, 23(9), 950.)
[4] Yang, W. H.; Xiao, G. M.; Kong, X. X. Appl. Chem. 2003, 20(4), 406 (in Chinese). (杨为华, 肖国民, 孔祥翔, 应用化学, 2003, 20(4), 406.)
[5] Dupont, J.; de Souza, R. F.; Suarez, P. A. Z. Chem. Rev. 2002, 102, 3667.
[6] Ye, H.; Wang, W.; Zhu, X.; Chen, W.; Xie, L.; Yuan, Y. Chin. J. Org. Chem. 2013, 33, 827 (in Chinese). (叶宏民, 王薇, 朱晓晓, 陈伟强, 谢莉莉, 袁耀锋, 有机化学, 2013, 33, 827.)
[7] Bian, L.; Zeng, X.; He, R.; Luo, C.; Lin, Z. Chin. J. Org. Chem. 2014, 34, 994 (in Chinese). (卞垒, 曾向潮, 何如, 罗创龙, 林志强, 有机化学, 2014, 34, 994.)
[8] Gao, X.; Fan, J.; Wang, X.; Zhang, Y. Acta Chim. Sinica 2013, 71, 1411 (in Chinese). (高霞, 樊静, 王小龙 张艳树, 化学学报, 2013, 71, 1411.)
[9] Bourissou, D.; Guerret, O.; Gabbai, F. P.; Bertrand, G. Chem. Rev. 2000, 100, 39.
[10] Climent, M. J.; Corma, A.; Iborra, S. RSC Adv. 2012, 2, 16.
[11] Domling, A. Chem. Rev. 2006, 106, 17.
[12] Cao, S.; Jing, Y.; Liu, Y.; Wan, J. Chin. J. Org. Chem. 2014, 34, 876 (in Chinese). (曹硕, 景艳锋, 刘云云, 万结平, 有机化学, 2014, 34, 876.)
[13] Tang, M.; Xing, D.; Cai, M.; Hu, W. Chin. J. Org. Chem. 2014, 34, 1268 (in Chinese). (唐敏, 邢栋, 蔡茂强, 胡文浩, 有机化学, 2014, 34 , 1268.)
[14] Radziszewski, B. Chem. Ber. 1882, 15, 1493.
[15] Japp, F.; Robinson, H. Chem. Ber. 1882, 15, 1268.
[16] Karimi-Jaberi, Z.; Barekat, M. Chin. Chem. Lett. 2010, 21(10), 1183.
[17] Joshi, R. S.; Mandhane, P. G.; Shaikh, M. U.; Kale, R. P.; Gill, C. H. Chin. Chem. Lett. 2010, 21(4), 429.
[18] Pasha, M. A.; Nizam, A. J. Saudi Chem. Soc. 2011, 15(1), 55
[19] Das, B.; Kashanna, J.; Kumar, R. A.; Jangili, P. Monatsh. Chem. 2013, 144 (2), 223.
[20] Sharma, S. D.; Hazarika, P.; Konwar, D. Tetrahedron Lett. 2008, 49(14), 2216.
[21] Reddy, M. V.; Jeong, Y. T. J. Fluorine Chem. 2012, 142, 45.
[22] Khosropour, A. R. Ultrason. Sonochem. 2008, 15(5), 659.
[23] Sangshetti, J. N.; Kokare, N. D.; Kotharkar, S. A.; Shinde, D. B. Chin. Chem. Lett. 2008, 19,762.
[24] Wang, L.-M.; Wang, Y.-H.; Tian, H.; Yao, Y.-F.; Shao, J.-H.; Liu, B. J. Fluorine Chem. 2006, 127(12), 1570.
[25] Shen, M.-G.; Cai, C.; Yi, W.-B. J. Fluorine Chem. 2008, 129(6), 541
[26] Karami, B.; Dehghani, F. M.; Eskandari, K. Croat. Chem. Acta 2012, 85(2), 147.
[27] Rajanarendar, E.; Murthy, K. R.; Reddy, M. N. Indian J. Chem. 2011, 50B, 926
[28] Kidwai, M.; Mothsra, P.; Bansal, V.; Somvanshi, R. K.; Ethayathulla, A. S.; Dey, S.; Singh, T. P. J. Mol. Catal. A: Chem. 2007, 265(1~2), 177.
[29] Wang, X. C.; Gong, H. P.; Quan, Z. J.; Li, L.; Ye, H. L. Chin. Chem. Lett. 2009, 20(1), 44
[30] Chary, M. V.; Keerthysri, N. C.; Vupallapati, S. V. N.; Lingaiah, N.; Kantevari, S. Catal. Commun. 2008, 9(10), 2013.
[31] Murthy, S. N.; Madhav, B.; Nageswar, Y. V. D. Tetrahedron Lett. 2010, 51(40), 5252.
[32] Ramesh, K.; Murthy, S. N.; Karnakar, K.; Nageswar, Y. V. D.; Vijayalakhshmi, K.; Devi, B. L. A. P.; Prasad, R. B. N. Tetrahedron Lett. 2012, 53(9), 1126.
[33] Samai, S.; Nandi, G. C.; Singh, P.; Singh, M.S. Tetrahedron 2009, 65(49), 10155.
[34] Nagargoje, D.; Mandhane, P.; Shingote, S.; Badadhe, P.; Gill, C. Ultrason. Sonochem. 2012 19, 94.
[35] Mahajan, A.; Aulakh, R. K.; Bedi , R. K.; Kumar, S.; Kumar, S.; Aswal, D. K. Synth. Met. 2012, 162, 58.
[36] Martorana, A.; Pace, A.; Buscemi, S.; Piccionello, A. P. Org. Lett. 2012, 14(13), 3240.
[37] Xue, W.-J.; Li, H.-Z.; Gao, F.-F.; Wu, A. Tetrahedron 2014, 70(2), 239.
[38] Zang, H.; Su, Q.; Mo, Y.; Cheng, B.-W.; Jun, S. Ultrason. Sonochem. 2010, 17(5), 749.
[39] Siddiqui, S. A.; Narkhede, U. C.; Palimkar, S. S.; Daniel, T.; Lahoti, R. J.; Srinivasan K. V. Tetrahedron 2005, 61(14), 3539.
[40] Xia, M.; Lu, Y.-D. J. Mol. Catal. A: Chem. 2007, 265(1~2), 205.
[41] Shaterian, H. R.; Ranjbar, M. J. Mol. Liq. 2011, 160 (1), 40.
[42] Zolfigol, M. A.; Khazaei, A.; Moosavi-Zare, A. R.; Zare, A.; Asgari, Z.; Khakyzadeh, V.; Hasaninejad, A. J. Ind. Eng. Chem. 2013, 19(3), 721.
[43] MaGee, D. I.; Bahramnejad, M.; Dabiri, M. Tetrahedron Lett. 2013, 54(21), 2591.
[44] Jourshari, M. S.; Mamaghani, M.; Shirini, F.; Tabatabaeian, K.; Rassa, M.; Langari, H. Chin. Chem. Lett. 2013, 24(11), 993.
[45] Heravi, M. M.; Derikvand, F.; Bamoharram, F. F. J Mol. Catal. A: Chem. 2007, 263(1~2), 112.
[46] Nagarapu, L.; Apuri, S.; Kantevari, S. J Mol. Catal. A: Chem. 2007, 266(1~2), 104.
[47] Fantini, M.; Zuliani, V.; Spotti, M. A.; Rivara, M. J. Comb. Chem. 2010, 12(1), 181.
[48] Heravi, M. M.; Bakhtiari, K.; Oskooie, H. A.; Taheri, S. J. Mol. Catal. A: Chem. 2007, 263(1~2), 279.
[49] Kannan, V.; Sreekumar, K. J. Mol. Catal. A: Chem. 2013, 376, 34.
[50] Kantevari, S.; Vuppalapati, S. V. N.; Biradar, D. O.; Nagarapu. L. J. Mol. Catal. A: Chem. 2007, 266(1~2), 109.
[51] Karimi, A. R.; Alimohammadi, Z.; Azizian, J.; Mohammadi, A. A.; Mohammadizadeh, M. R. Catal. Commun. 2006, 7(9), 728.
[52] Niknam, K.; Deris, A.; Naeimi, F.; Majleci, F. Tetrahedron Lett. 2011, 52(36), 4642.
[53] Mukhopadhyay, C.; Tapaswi, P. K.; Drew, M. G. B. Tetrahedron Lett. 2010, 51(30), 3944.
[54] Sivakumar, K.; Kathirvel, A.; Lalitha, A. Tetrahedron Lett. 2010, 51(22), 3018.
[55] Gupta, P.; Paul, S. J. Mol. Catal. A: Chem. 2012, 352, 75
[56] Mohammadi, A.; Keshvari, H.; Sandaroos, R.; Maleki, B.; Rouhi, H.; Moradi, H.; Sepehr, Z.; Damavandi, S. Appl. Catal., A: Gen. 2012, 429~430, 73.
[57] Mekheimer, R. A.; Hameed, A. M. A.; Mansour, S. A. A.; Sadek, K. U. Chin. Chem. Lett. 2009, 20(7), 812.
[58] Xiao, L. W.; Gao, H. J.; Kong, J.; Liu, G. X.; Li, L. L.; Duan, J. D. Chin. J. Org. Chem. 2014, 34(12), 2511 (in Chinese). (肖立伟, 高红杰, 孔洁, 刘光仙, 李玲玲, 段敬丹, 有机化学, 2014, 34(12), 2511.)
[59] Safari, J.; Zarnegar, Z. C. R. Chim. 2013, 16(10), 920.
[60] Safari, J.; Zarnegar, Z. Ultrason. Sonochem. 2013, 20(2), 740.
[61] Safari, J.; Gandomi-Ravandi, S.; Akbari, Z. J. Adv. Res., 2013, 4(6), 509.
[62] Teimouri, A.; Chermahini, A. N. J. Mol. Catal. A: Chem. 2011, 346(1~2), 39.
[63] Ray, S.; Das, P.; Bhaumik, A.; Dutta, A.; Mukhopadhyay, C. Appl. Catal., A: Gen. 2013, 458, 183.
[64] Keivanloo, A.; Bakherad, M.; Imanifar, E.; Mirzaee, M. Appl. Catal., A: Gen. 2013, 467, 291.
[65] Mirjalili, B. F.; Bamoniri, A. H.; Zamani, L. Sci. Iran. 2012, 19(3), 565.
[66] Aziizi, N.; Manochehri, Z.; Nahayi, A.; Torkashvand, S. J. Mol. Liq. 2014, 196, 153.
[67] Rostamnia, S.; Zabardasti, A. J. Fluorine Chem. 2012, 144, 69.
[68] Rajaguru, K.; Suresh, R.; Mariappan, A.; Muthusubramanian, S.; Bhuvanesh, N. Org. Lett. 2014, 16(3), 744.
[69] Pandya, A. N.; Agrawal, D. K. Tetrahedron Lett. 2014, 55(10), 1835.
[70] Mamedov, V. A.; Zhukova, N. A.; Beschastnova, T. N.; Gubaidullin, A. T.; Rakov, D. V.; Rizvanov, I. K. Tetrahedron Lett. 2011, 52(33), 4280.
[71] Li, B.; Gu, Q.; He, Y.; Zhao, T.; Wang, S.; Kang, J.; Zhang, Y. C. R. Chim. 2012, 15(9), 784.
[72] Mlostoń, G.; Obijalska, E.; Heimgartner, H. J. Fluorine Chem. 2011, 132(11), 951.
[73] Liu, X.; Wang, D.; Chen, B. Tetrahedron 2013, 69(45), 9417.
[74] Tang, D.; Wu, P.; Liu, X.; Chen, Y.-X.; Guo, S.-B.; Chen, W.-L.; Li, J.-G.; Chen, B.-H. J. Org. Chem. 2013, 78(6), 2746.
[75] Guo, C.; Zhang, C.; Li, X.; Li, W.; Xu, Z.; Bao, L.; Ding, Y.; Wang, L.; Li, S. Bioorg. Med. Chem. Lett. 2013, 23(21), 5850.
[76] Pusch, S.; Opatz, T. Org. Lett. 2014, 16, 5430.
[77] Mehdi, A.; Samira, A.; Shahzad, F.; Jafar, A. D.; Peiman, M. Synlett 2009, 3263.
[78] Siamaki, A. R.; Arndtsen, B. A. J. Am. Chem. Soc. 2006, 128, 6050.
[79] Jiang, Z; Lu, P.; Wang, Y. Org. Lett. 2012, 14(24), 6266.
[80] Chen, C.-Y.; Hu, W.-P.; Yan, P.-C.; Senadi, G. C.; Wang, J.-J. Org. Lett. 2013, 15(24), 6116.
/
| 〈 |
|
〉 |