

氧化型导向基诱导的C—H键官能团化
收稿日期: 2014-12-30
修回日期: 2015-02-13
网络出版日期: 2015-02-14
基金资助
国家自然科学基金(No. 21202184)资助项目.
C—H Functionalization Induced by the Oxidizing Directing Group
Received date: 2014-12-30
Revised date: 2015-02-13
Online published: 2015-02-14
Supported by
Project supported by the National Natural Science Foundation of China (No. 21202184).
胡志勇 , 童晓峰 , 刘桂霞 . 氧化型导向基诱导的C—H键官能团化[J]. 有机化学, 2015 , 35(3) : 539 -555 . DOI: 10.6023/cjoc201412050
Transition-metal catalyzed C—H functionalization is a straightforward and efficient way to construct C—C and C—X bonds. However, these transformations usually require stioichiometric or excess amount of external oxidants to oxidize low valent metal and regenerate the active catalytic species. The use of the oxidizing directing group, which contains some special group acting as the internal oxidant, can avoid the troubles arousing from the external oxidants and make the reaction process more simple and efficient. Recently, increasing number of novel oxidizing directing groups have been designed and diverse kinds of reactions have been developed. This approach can be used to synthesize kinds of heterocyles or highly functionalized products under external oxidant free conditions.
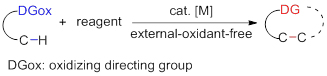
[1] (a) Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068. (b) Li, B.-J.; Shi, Z.-J. Chem. Soc. Rev. 2012, 41, 5588. (c) Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236. (d) Song, G. Y.; Wang, F.; Li, X. W. Chem. Soc. Rev. 2012, 41, 3651.
[2] Lewis, J. C.; Bergman, R. G.; Ellman, J. Acc. Chem. Res. 2008, 41, 1013.
[3] Arockiam, P. B.; Bruneau, C.; Dixneuf, P. H. Chem. Rev. 2012, 112, 5879.
[4] Engle, K. M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q. Acc. Chem. Res. 2012, 45, 788.
[5] Kozhushkov, S. I.; Ackermann, L. Chem. Sci. 2013, 4, 886.
[6] Frasco, D. A.; Lilly, C. P.; Boyle, P. D.; Ison, E. A. ACS Catal. 2013, 3, 2421.
[7] Stuart, D. R.; Mégan, B.-L.; Burgess, K. M. N.; Fagnou, K. J. Am. Chem. Soc. 2008, 130, 16474.
[8] Li, G.; Leow, D.; Wan, L.; Yu, J.-Q. Angew. Chem., Int. Ed. 2013, 52, 1245.
[9] Boele, M. D. K.; Strijdonck, G. P. F.; Vries, A. H. M.; Kamer, P. C. J.; Vries, J. G.; Leeuwen, P. W. N. M. J. Am. Chem. Soc. 2002, 124, 1586.
[10] Cheng, X.-F.; Li, Y.; Su, Y.-M.; Yin, F.; Wang, J.-Y.; Sheng, J.; Vora, H. U.; Wang, X.-S.; Yu J.-Q. J. Am. Chem. Soc. 2013, 135, 1236.
[11] (a) Tsai, A.; Tauchert, M.; Bergman, E.; Ellman, J. J. Am. Chem. Soc. 2011, 133, 1248. (b) Li, Y.; Zhang, X.; Chen, K.; He, K.; Shi, Z. Org. Lett. 2012, 14, 636. (c) Feng, C.; Feng, D.; Loh, T.-P. Org. Lett. 2013, 15, 3670.
[12] (a) Wendlandt, A. E.; Suess, A. M.; Stahl, S. S. Angew. Chem., Int. Ed. 2011, 50, 11062. (b) Wang, D.-H.; Engle, K. M.; Shi, B.-F.; Yu, J.-Q. Science 2010, 327, 315.
[13] (a) Patureau, F. W.; Glorius, F. Angew. Chem. 2011, 123, 2021. (b) Huang, H.; Ji, X.; Wu, W.; Jiang, H. Chem. Soc. Rev. 2015, 44, 1155. (c) Mo, J.; Wang, L.; Liu, Y.; Cui, X. Synthesis 2015, 47, 439.
[14] Ng, K. H.; Chan, A. S. C.; Yu, W. Y. J. Am. Chem. Soc. 2010, 132, 12862.
[15] Yoo, E. J.; Ma. S.; Mei, T.-S.; Chan, K. S. L.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 7652.
[16] Dong, Z.; Dong, G. J. Am. Chem. Soc. 2013, 135, 18350.
[17] Patel, P.; Chang, S. Org. Lett. 2014, 16, 3328.
[18] Guimond, N.; Gouliaras, C.; Fagnou, K. J. Am. Chem. Soc. 2010, 132, 6908.
[19] (a) Guimond, N.; Gorelsky, S. I.; Fagnou, K. J. Am. Chem. Soc. 2011, 133, 6449. (b) Xu, L.; Zhu, Q.; Huang, G.; Cheng, B.; Xia, Y. J. Org. Chem. 2012, 77, 3017.
[20] Rakshit, S.; Grohmann, C.; Besset, T.; Glorius, F. J. Am. Chem. Soc. 2011, 133, 2350.
[21] Xu, X.; Liu, Y.; Park, C.-M. Angew. Chem. 2012, 124, 9506.
[22] Davis, T. A.; Hyster, T. K.; Rovis T. Angew. Chem., Int. Ed. 2013, 52, 14181.
[23] Yu, D.-G.; de Azambuja, F.; Gensch, T.; Daniliuc, C. G.; Glorius, F. Angew. Chem., Int. Ed. 2014, 53, 9650.
[24] Wang, H.; Grohmann, C.; Nimphius, C.; Glorius, F. J. Am. Chem. Soc. 2012, 134, 19592.
[25] Presset, M.; Oehlrich, D.; Rombouts, F.; Molander, G. A. Org. Lett. 2013, 15, 1528.
[26] Huckins, J. R.; Bercot, E. A.; Thiel, O. R.; Hwang, T.-L.; Bio, M. M. J. Am. Chem. Soc. 2013, 135, 14492.
[27] Li, B.; Feng, H. L.; Xu ,S.; Wang, B. Chem. Eur. J. 2011, 17, 12573.
[28] Ackermann, L.; Fenner, S. Org. Lett. 2011, 13, 6548.
[29] Li, B.; Ma, J.; Wang, N.; Feng, H.; Xu, S.; Wang, B. Org. Lett. 2012, 14, 736.
[30] Fukui, Y.; Liu, P.; Liu, Q.; He, Z.-T.; Wu, N.-Y.; Tian, P.; Lin, G.-Q. J. Am. Chem. Soc. 2014, 136, 15607.
[31] Wang, H.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 7318
[32] Zeng, R.; Wu, S.; Fu, C. L.; Ma, S. J. Am. Chem. Soc. 2013, 135, 18284.
[33] Hyster, T. K.; Ruhl, K. E.; Rovis, T. J. Am. Chem. Soc. 2013, 135, 5364.
[34] Cui, S.; Zhang, Y; Wang, D.; Wu, Q. Chem. Sci. 2013, 4, 3912.
[35] (a) Hyster, T. K.; Rovis, T. Synlett 2013, 1842. (b) Cui, S.; Zhang, Y.; Wu, Q. Chem. Sci. 2013, 4, 3421.
[36] Zhang, Y.; Zheng, J.; Cui, S. J. Org. Chem. 2014, 79, 6490.
[37] Liu, G.; Shen, Y.; Zhou, Z.; Lu, X. Angew. Chem., Int. Ed. 2013, 52, 6033.
[38] Shen, Y.; Liu, G.; Zhou, Z.; Lu, X. Org. Lett. 2013, 15, 3366.
[39] Zhou, Z.; Liu, G.; Shen, Y.; Lu, X. Org. Chem. Front. 2014, 1, 1161.
[40] Hu, F.; Xia, Y.; Ye, F.; Liu, Z.; Ma, C.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2014, 53, 1364.
[41] Zhang, H.; Wang, K.; Wang, B.; Yi, H.; Hu, F.; Li, C.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2014, 53, 13234.
[42] Piou, T.; Rovis, T. J. Am. Chem. Soc. 2014, 136, 11292.
[43] Li, X. G.; Liu, K.; Zou, G.; Liu, P. N. Adv. Synth. Catal. 2014, 356, 1496.
[44] Hyster, T. K.; Knörr, L.; Ward, T. R.; Rovis, T. Science 2012, 338, 500.
[45] Ye, B.; Cramer, N. Science 2012, 338, 504.
[46] Ye, B.; Donets, P. A.; Cramer, N. Angew. Chem., Int. Ed. 2014, 53, 507.
[47] Ye, B.; Cramer, N. J. Am. Chem. Soc. 2013, 135, 636.
[48] Ye, B.; Cramer, N. Angew. Chem., Int. Ed. 2014, 53, 7896.
[49] Too, P. C.; Wang Y.-F.; Chiba S. S. Org. Lett. 2010, 12, 5688.
[50] Zhang, X.; Chen, D.; Zhao, M.; Zhao J.; Jia, A.; Li, X. Adv. Synth. Catal. 2011, 353, 719.
[51] Too, P. C.; Noji, T.; Lim, Y. J.; Li, X.; Chiba, S. Synlett 2011, 2789.
[52] Hyster, T. K.; Rovis, T. Chem. Commun. 2011, 47, 11846.
[53] Chinnagolla, R. K.; Pimparkar, S.; Jeganmohan, M. Org. Lett. 2012, 14, 3032.
[54] Kornhaaß, C.; Li J.; Ackermann, L. J. Org. Chem. 2012, 77, 9190.
[55] Neely, J. M.; Rovis, T. J. Am. Chem. Soc. 2013, 135, 66.
[56] Neely, J. M.; Rovis, T. J. Am. Chem. Soc. 2014, 136, 2735.
[57] Zhao, D.; Lied, F.; Glorius, F. Chem. Sci. 2014, 5, 2869.
[58] Cho, S. H.; Hwang, S. J.; Chang, S. J. Am. Chem. Soc. 2008, 130, 9254.
[59] Liu, W.; Li, Y. H.; Kuang, C. X. Org. Lett. 2013, 15, 2342.
[60] Wu, J.; Cui, X.; Chen, L.; Jiang, G.; Wu, Y. J. Am. Chem. Soc. 2009, 131, 13888.
[61] Huang, X.; Huang, J.; Du, C.; Zhang, X.; Song, F.; You, J. Angew. Chem., Int. Ed. 2013, 52, 12970.
[62] Zhang, X.; Qi, Z.; Li, X. Angew. Chem., Int. Ed. 2014, 53, 10794.
[63] Zhao, D.; Shi, Z.; Glorius, F. Angew. Chem., Int. Ed. 2013, 52, 12426.
[64] Zheng, L.; Hua, R. Chem. Eur. J. 2014, 20, 2352.
[65] Muralirajana, K.; Cheng, C. H. Adv. Synth. Catal. 2014, 356, 1571.
[66] Zhang, Z.; Jiang, H.; Huang, Y. Org. Lett. 2014, 16, 5976.
[67] Chuang, S.-C.; Gandeepan, P.; Cheng, C.-H. Org. Lett. 2013, 15, 5750.
[68] Liu, B.; Song, C.; Sun, C.; Zhou, S.; Zhu, J. J. Am. Chem. Soc. 2013, 135, 16625.
[69] Wang, C.; Huang, Y. Org. Lett. 2013, 15, 5294.
[70] Zhang, Q.-R.; Huang, J.-R.; Zhang, W.; Dong, L. Org. Lett. 2014, 16, 1684.
[71] Yadav, M. R.; Rit, R. K.; Shankar, M.; Sahoo, A. K. J. Org. Chem. 2014, 79, 6123.
[72] Gerfaud, T.; Neuville, L.; Zhu, J. Angew. Chem., Int. Ed. 2009, 48, 572.
[73] Tan, Y.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 3676.
[74] Chiba, S. Chem. Lett. 2012, 41, 1554.
/
| 〈 |
|
〉 |