

环状脱氢氨基酸及其衍生物的不对称催化氢化研究
收稿日期: 2015-02-09
修回日期: 2015-02-28
网络出版日期: 2015-03-03
基金资助
国家自然科学基金(No. 21202096)资助项目.
Asymmetric Hydrogenation of Cyclic Dehydroamino Acids and Their Derivatives
Received date: 2015-02-09
Revised date: 2015-02-28
Online published: 2015-03-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21202096).
王英杰 , 张振锋 , 张万斌 . 环状脱氢氨基酸及其衍生物的不对称催化氢化研究[J]. 有机化学, 2015 , 35(3) : 528 -538 . DOI: 10.6023/cjoc201502017
Chiral cyclic amino acids, possessing the unique structure and special properties, can be found in various chiral drugs and chiral catalysts as structural motifs. Transition-metal-catalyzed asymmetric hydrogenation, which has the advantages of high efficiency, environmental friendliness and atom economy, becomes the preferred method for the syntheses of such compounds. The related works on this field are reviewed for the first time in this paper. The catalytic asymmetric hydrogenation of various types of cyclic α- and β-dehydroamino acids and their derivatives is introduced, and the advantages and disadvantages of different kinds of transition-metal catalysts (including complexes of Ru, Rh, and Ir) are discussed. Thus a perspective is proposed for the future development.
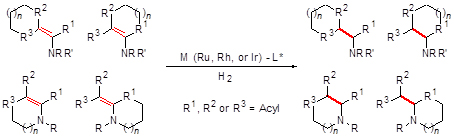
[1] (a) de Vries, J. G.; Elsevier, C. J. Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, Germany, 2007. (b) Ma, Y.; Zhang, Y. J.; Zhang, W. Chin. J. Org. Chem. 2007, 27, 289 (in Chinese). (马元辉, 张勇健, 张万斌, 有机化学, 2007, 27, 289.) (c) Blaser, H.-U.; Federsel, H.-J. Asymmetric Catalysis on Industrial Scale, 2nd., Wiley-VCH, Weinheim, Germany, 2010. (d) Xie, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2012, 70, 1427 (in Chinese). (谢建华, 周其林, 化学学报, 2012, 70, 1427.) (e) Etayo, P.; Vidal-Ferran, A. Chem. Soc. Rev. 2013, 42, 728.
[2] (a) Dydio, P.; Rubay, C.; Gadzikwa, T.; Lutz, M.; Reek, J. N. H. J. Am. Chem. Soc. 2011, 133, 17176. (b) van Leeuwen, P. W. N. M.; Rivillo, D.; Raynal, M.; Freixa, Z. J. Am. Chem. Soc. 2011, 133, 18562. (c) Imamoto, T.; Tamura, K.; Zhang, Z.; Horiuchi, Y.; Sugiya, M.; Yoshida, K.; Yanagisawa, A.; Gridnev, I. D. J. Am. Chem. Soc. 2012, 134, 1754. (d) Wang, Q.; Huang, W.; Yuan, H.; Cai, Q.; Chen, L.; Lv, H.; Zhang, X. J. Am. Chem. Soc. 2014, 136, 16120. (e) Yang, P.; Xu, H.; Zhou, J. Angew. Chem., Int. Ed. 2014, 53, 12210. (f) Molinaro, C.; Scott, J. P.; Shevlin, M.; Wise, C.; Ménard, A.; Gibb, A.; Junker, E. M.; Lieberman, D. J. Am. Chem. Soc. 2015, 137, 999. (g) Gao, M.; Meng, J.-J.; Lv, H.; Zhang, X. Angew. Chem., Int. Ed. 2015, 54, 1885.
[3] Bruneau, C.; Renaud, J.-L.; Jerphagnon, T. Coord. Chem. Rev. 2008, 252, 532.
[4] Buser, H. P.; Pugin, B.; Spindler, F.; Sutter, M. Tetrahedron 1991, 47, 5709.
[5] Armstrong, III, J. D.; Eng, K. K.; Keller, J. L.; Purick, R. M.; Hartner, Jr., F. W.; Choi, W.-B.; Askin, D.; Volante, R. P. Tetrahedron Lett. 1994, 35, 3239.
[6] Foti, C. J.; Comins, D. L. J. Org. Chem. 1995, 60, 2656.
[7] Wilkinson, T. J.; Stehle, N. W.; Beak, P. Org. Lett. 2000, 2, 155.
[8] Lim, S. H.; Ma, S.; Beak, P. J. Org. Chem. 2001, 66, 9056.
[9] Nicolaou, K. C.; Shi, G.-Q.; Namoto, K.; Bernal, F. Chem. Commun. 1998, 1757.
[10] (a) Kuwano, R.; Karube, D.; Ito, Y. Tetrahedron Lett. 1999, 40, 9045. (b) Kuwano, R.; Ito, Y. J. Org. Chem. 1999, 64, 1232.
[11] Kuwano, R.; Sato, K.; Kurokawa, T.; Karube, D.; Ito, Y. J. Am. Chem. Soc. 2000, 122, 7614.
[12] Mrši?, N.; Jerphagnon, T.; Minnaard, A. J.; Feringa, B. L.; de Vries, J. G. Tetrahedron: Asymmetry 2010, 21, 7.
[13] Maj, A. M.; Suisse, I.; Méliet, C.; Agbossou-Niedercorn, F. Tetrahedron: Asymmetry 2010, 21, 2010.
[14] Baeza, A.; Pfaltz, A. Chem. Eur. J. 2010, 16, 2036.
[15] (a) Imamoto, T.; Watanabe, J.; Wada, Y.; Masuda, H.; Yamada, H.; Tsuruta, H.; Matsukawa, S.; Yamaguchi, K. J. Am. Chem. Soc. 1998, 120, 1635. (b) Ohashi, A.; Imamoto, T. Org. Lett. 2001, 3, 373. (c) Ohashi, A.; Kikuchi, S.-I.; Yasutake, M.; Imamoto, T. Eur. J. Org. Chem. 2002, 2535. (d) Oohara, N.; Katagiri, K.; Imamoto, T. Tetrahedron: Asymmetry 2003, 14, 2171.
[16] Imamoto, T.; Oohara, N.; Takahashi, H. Synthesis 2004, 1353.
[17] Imamoto, T.; Yashio, K.; Crépy, K. V. L.; Katagiri, K.; Takahashi, H.; Kouchi, M.; Gridnev, I. D. Organometallics 2006, 25, 908.
[18] Gridnev, I. D.; Imamoto, T.; Hoge, G.; Kouchi, M.; Takahashi, H. J. Am. Chem Soc. 2008, 130, 2560.
[19] Wada, Y.; Imamoto, T.; Tsuruta, H.; Yamaguchi, K.; Gridnev, I. D. Adv. Synth. Catal. 2004, 346, 777.
[20] (a) Stephan, M.; Šterk, D.; Mohar, B. Adv. Synth. Catal. 2009, 351, 2779. (b) Zupan?i?, B.; Mohar, B.; Stephan, M. Org. Lett. 2010, 12, 1296. (c) Zupan?i?, B.; Mohar, B.; Stephan, M. Org. Lett. 2010, 12, 3022.
[21] Kuwano, R.; Sawamura, M.; Ito, T. Bull. Chem. Soc. Jpn. 2000, 73, 2571.
[22] Evans, D. A.; Michael, F. E.; Tedrow, J. S.; Campos, K, R. J. Am. Chem Soc. 2003, 125, 3534.
[23] Reetz, M. T.; Li, X. Synthesis 2005, 3183.
[24] Jiang, X.-B.; van den Berg, M.; Minnaard, A. J.; Feringa, B. L.; de Vries, J. G. Tetrahedron: Asymmetry 2004, 15, 2223.
[25] Tang, W.; Wu, S.; Zhang, X. J. Am. Chem. Soc. 2003, 125, 9570.
[26] Kajiwara, T.; Konishi, T.; Yamano, M. Catal. Sci. Technol. 2012, 2, 2146.
[27] Huang, K.; Guan, Z.-H.; Zhang, X. Tetrahedron Lett. 2014, 55, 1686.
[28] Wu, H.-P.; Hoge, G. Org. Lett. 2004, 6, 3645.
[29] Enthaler, S.; Erre, G.; Junge, K.; Holz, J.; Börner, A.; Alberico, E.; Nieddu, I.; Gladiali, S.; Beller, M. Org. Proc. Res. Dev. 2007, 11, 568.
[30] Pousset, C.; Callens, R.; Marinetti, A.; Larchevêque, M. Synlett 2004, 2766.
[31] Karamé, I.; Nemra-Baydoun, G.; Abdallah, R.; Kanj, A.; Börner, A. Synth. Commun. 2011, 41, 583.
[32] Zhang, Y. J.; Park, J. H.; Lee, S.-G. Tetrahedron: Asymmetry 2004, 15, 2209.
[33] Lei, A.; Chen, M.; He, M.; Zhang, X. Eur. J. Org. Chem. 2006, 4343.
[34] Bisset, A. A.; Shiibashi, A.; Desmond, J. L.; Dishington, A.; Jones, T.; Clarkson, G. J.; Ikariya, T.; Wills, M. Chem. Commun. 2012, 48, 11978.
[35] Enthaler, S.; Erre, G.; Junge, K.; Schröder, K.; Addis, D.; Michalik, D.; Hapke, M.; Redkin, D.; Beller, M. Eur. J. Org. Chem. 2008, 3352.
/
| 〈 |
|
〉 |