

新型多环芳烃Bisanthene衍生物的合成研究进展
收稿日期: 2015-01-20
修回日期: 2015-03-02
网络出版日期: 2015-03-09
基金资助
国家自然科学基金(No. 21302043), 河南工业大学科技创新人才培育计划(No. 2013CXRC10)和河南工业大学校高层次人才基金(No. 2012BS051)资助项目.
Synthesis of Bisanthene-Based Polycyclic Aromatic Hydrocarbons
Received date: 2015-01-20
Revised date: 2015-03-02
Online published: 2015-03-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21302043); Plan For Scientific Innovation Talent of Henan University of Technology (No. 2013CXRC10); High-Level talent foundation of Henan University of Technology (No. 2012BS051).
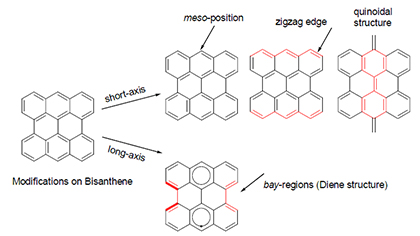
多环芳烃共轭体系作为一种新型的有机光电材料, 在有机电子学领域占据着举足轻重的地位. 其家族成员Bisanthene具有优异的特性, 拥有相对较小的分子能级及平面共轭双烯结构, 可以进行化学修饰来合成多种新型多环芳烃. 根据Bisanthene结构的特殊性, 可以对其短轴纵向修饰以及长轴横向修饰, 从而扩大其π电子共轭体系, 提高分子的溶解性及稳定性. 综述了Bisanthene衍生物的合成方法及性质的研究进展, 并对今后的研究方向进行了展望.
关键词: 多环芳烃; Bisanthene; π-共轭; 化学修饰; 有机光电材料
李金玲 , 谢宝粘 , 彭进 . 新型多环芳烃Bisanthene衍生物的合成研究进展[J]. 有机化学, 2015 , 35(7) : 1441 -1450 . DOI: 10.6023/cjoc201501025
Polycyclic aromatic hydrocarbons (PAHs) with extended π-conjugation play a significant role as novel organic optoelectronic materials. As a family member of PAHs, bisanthene possesses unique properties and features such as small band gap and planar diene structure, which benefits it as a key building block to construct new PAHs by appropriate design and chemical modification. For example, the modifications along with its short-axis and long-axis could not only enlarge its π-conjugated path route, but also improve the solubility and stability. The recent research progress in the synthesis and properties of bisanthene derivatives is summarized, and the future research of this field is prospected.

[1] (a) Clar, E. Aromatische Kohlenwasserstoffe-Polycyclische Systeme, Berlin, Springer, 1952.(b) Zander, M. Polycyclische Aromaten-Kohlenwasserstoffe und Fullerene, Stuttgart, Teubner, 1995.(c) Clar, E. Polycyclic Hydrocarbons, Vol. I/II, Academic Press, New York, 1964. (d) Clar, E. The Aromatic Sextet, Wiley-VCH, London, 1972. (e) Clar, E.; Schmidt, W. Tetrahedron 1979, 35, 2673. (f) Zander, M. Handbook of Polycyclic Aromatic Hydrocarbons, Marcel Dekker, New York, 1983. (g) Harvey, R. G. In Polycyclic Aromatic Hydrocarbons, Wiley-VCH, Weinheim, 1997.
[2] Stein. S. E.; Brown, R. L. J. Am. Chem. Soc. 1987, 109, 3721.
[3] (a) Ye, H.-Y.; Wen, L.; Li, W.-S. Chin. J. Org. Chem. 2012, 32, 266 (in Chinese).(叶怀英, 李文, 李维实, 有机化学, 2012, 32, 266.)(b) Qu, H.-M.; Jiang, L.-L.; Chen, T; Tang, J.-K. Chin. J. Org. Chem. 2014, 34, 1061 (in Chinese).(曲红梅, 蒋丽丽, 陈涛, 唐建可, 有机化学, 2014, 34, 1061.)
[4] (a) Bendikov, M.; Wudl, F.; Perepichka, D. F. Chem. Rev. 2004, 104, 4891.(b) Anthony, J. E. Chem. Rev. 2006, 106, 5028.(c) Murphy, A. R.; Fréchet, J. M. J. Chem. Rev. 2007, 107, 1066. (d) Wu, J.; Pisula, W.; Müllen, K. Chem. Rev. 2007, 107, 718.(e) Luo, J.; Xu, M.; Li, R.; Huang, K.-W.; Jiang, C.; Qi, Q.; Zeng, W.; Zhang, J.; Chi, C.; Wang, P.; Wu, J. J. Am. Chem. Soc. 2014, 136 , 265.
[5] (a) Wang, C. L.; Dong, H. L.; Hu, W. P.; Liu, Y. Q.; Zhu, D. B. Chem. Rev. 2012, 112, 2208.(b) Di, C.; Zhang, F.; Zhu, D. B. Adv. Mater 2013, 25, 313.(c) Thorlet, K. J.; Anthonyl, J. E. Isr. J. Chem. 2014, 54, 642.(d) Wang, H. L.; Zhang, L. S.; Chen, Z. G.; Hu, J. Q.; Li, S. J.; Wang, Z. H.; Liu, J. S.; Wang, X. C. Chem. Soc. Rev. 2014, 43, 5234
[6] (a) Anthony, J. E.; Eaton, D. L.; Parkin, S. R. Org. Lett. 2002, 4, 15.(b) Anthony, J. E. Angew. Chem., Int. Ed. 2008, 47, 452.(c) Kim, D. H.; Lee, D. Y.; Lee, H. S.; Lee, W. H.; Kim, Y. H.; Han, J. I.; Cho, K. Adv. Mater. 2007, 19, 678.(d) Wilson, M. W. B.; Rao, A.; Ehrler, B.; Friends, R. H. Acc. Chem. Res. 2013, 46, 1330.
[7] (a) Ling, M.; Erk, P.; Gomez, M.; Koenemann, M.; Locklin, J.; Bao, Z. Adv. Mater. 2007, 19, 1123.(b) Weitz, R. T.; Amsharov, K.; Zschieschang, U.; Villas, E. B.; Goswami, D. K.; Burghard, M.; Dosch, H.; Jansen, M.; Kern, K.; Klauk, H. J. Am. Chem. Soc. 2008, 130, 4637.(c) Piliego, C.; Jarzab, D.; Gigli, G.; Chen, Z.; Facchetti, A.; Loi, M. A. Adv. Mater. 2009, 21, 1573.(d) Liu, C. M.; Xiao, C. Y.; Li, Y.; Hu, W. P.; Li, Z, B.; Wang, Z. H. Chem. Commun. 2014, 50, 12462.
[8] Sun, Z.; Zeng, Z.; Wu, J. Acc. Chem. Res. 2014, 47, 2582.
[9] Kuroda, H. J. Chem. Phys. 1960, 1856.
[10] Clar, E. Chem. Ber. 1948, 81, 52.
[11] Sa?di-Besbes, S.; Grelet, É.; Bock, H. Angew. Chem., Int. Ed. 2006, 45, 1783.
[12] Zhang, K.; Huang, K.-W.; Li, J. L.; Chi, C.; Wu, J. Org. Lett. 2009, 11 ,4854.
[13] Li, J. L.; Zhang, K.; Huang, K.-W.; Chi, C.; Wu, J. J. Org. Chem. 2010, 75, 856.
[14] Kuroda, H. J. Chem. Phys. 1960, 33, 1586.
[15] Arabei, S. M.; Pavich, T. A. J. Appl. Spectrosc. 2000, 67, 23
[16] Hirao, Y.; Konishi, A.; Matsumoto, K.; Kurata, H.; Kubo, T. International Conference of Computational Methods in Sciences and Engineering 2009, Eds.: Simos, T. E.; Maroulis. G., American Institute of Physics, Rhodes, 2012, 1504, pp. 863~866.
[17] Jiang, D.-E.; Sumpter, B. G.; Dai, S. J. Chem. Phys. 2007, 127, 124703.
[18] Jiang, D.-E.; Dai, S. Chem. Phys. Lett. 2008, 466, 72.
[19] Maulding, D. R. J. Org. Chem. 1970, 35, 1221.
[20] Yao, J.; Chi, C.; Wu, J.; Loh, K. Chem. Eur. J. 2009, 15, 9299.
[21] West, R.; Jorgenson, J.; Stearley, K.; Calabrese, J. J. Chem. Soc., Chem. Commun. 1991, 1234.
[22] Takahashi, K.; Suzuki, T.; Akiyama, K.; Ikegami, Y.; Fukazawa, Y. J. Am. Chem. Soc. 1991, 113, 4576.
[23] Takahashi, T.; Matsuoka, K.; Takimiya, K.; Otsubo, T.; Aso, Y. J. Am. Chem. Soc. 2005, 127, 8928.
[24] Dumur, F.; Gautier, N.; Gallego-Planas, N.; Sahin, Y.; Levillain, E.; Mercier, N.; Hudhomme, P.; Masino, M.; Girlando, A.; Lloveras, V.; Vidal-Gancedo, J.; Veciana, J.; Rovira, C. J. Org. Chem. 2004, 69, 2164.
[25] Fort, E. H.; Donovan, P. M.; Scott, L. T. J. Am. Chem. Soc. 2009, 131, 16006.
[26] Fort, E. H.; Scott, L. T. Angew. Chem., Int. Ed. 2010, 49, 6626.
[27] Fort, E. H.; Jeffreys, M. S.; Scott, L. T. Chem. Commun. 2012, 48, 8102.
[28] Fort, E. H.; Scott, L. T. Org. Biomol. Chem. 2012, 10, 5747.
[29] Li, J. L.; Jiao, C. J.; Huang, K.-W.; Wu, J. Chem. Eur. J. 2011, 17,14672.
[30] (a) Clar, E. Nature, London 1948, 161, 238.(b) Clar, E. Chem. Ber. 1949, 82, 55.
[31] Li, J. L.; Chang, J. J.; Tan, P. H. S.; Jiang, H.; Chen, X. D.; Chen, Z. K.; Zhang, J.; Wu, J. Chem. Sci. 2012, 3, 846.
[32] Chang, J.; Li, J.; Chang, K. L.; Zhang, J.; Wu, J. RSC Adv. 2013, 3, 8721.
[33] Dral, P. O.; Kivala, M.; Clark, T. J. Org. Chem. 2013, 78, 1894.
[34] (a) Jiang, W.; Qian, H. L.; Li, Y.; Wang, Z. H. J. Org. Chem. 2008, 73, 7369. (b) Wei, J.; Han, B.; Guo, Q.; Shi, X.; Wang, W.; Wei, N. Angew. Chem., Int. Ed. 2010, 49, 8209. (c) Jiang, W.; Zhou, Y.; Geng, H.; Jiang, S. D.; Yan, S. K.; Hu, W. P.; Wang, Z. H.; Shuai, Z. G.; Pei, J. J. Am. Chem. Soc. 2011, 133, 1.
[35] Service, R. F. Science 2011, 332, 293.
/
| 〈 |
|
〉 |