

穿心莲内酯抗肿瘤作用衍生物的合成研究进展
收稿日期: 2015-01-21
修回日期: 2015-03-05
网络出版日期: 2015-03-09
基金资助
国家自然科学基金(No. 30973621)资助项目.
Recent Progress in Synthesis of Andrographolide Derivatives with Anti-tumor Activities
Received date: 2015-01-21
Revised date: 2015-03-05
Online published: 2015-03-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 30973621).
彭宇然 , 孙亦诚 , 王德才 , 韦萍 , 欧阳平凯 , 周国春 . 穿心莲内酯抗肿瘤作用衍生物的合成研究进展[J]. 有机化学, 2015 , 35(7) : 1451 -1468 . DOI: 10.6023/cjoc201501027
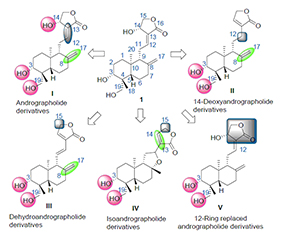
As one of the major active constituents in Andrographis panniculata (Burm. f.) Nees of Acanthaceae family, andrographolide is proved to possess a wide range of biological activities. In recent years, numerous studies show that the structural modification of andrographolide could enhance its anti-tumor activities. Therefore, according to the structural properties of andrographolide derivatives, five types of derivatives classified by different basic skeletons are denoted as the skeleton of andrographolide derivatives, the skeleton of 14-deoxyandrographolide derivatives, the skeleton of dehydroandrographolide derivatives, the skeleton of isoandrographolide derivatives and the skeleton of 12-ring replaced andrographolide derivatives. On this basis, the synthesis and anti-tumor activities of andrographolide derivatives are reviewed, and the structure activity relationship are also summarized preliminarily. The future trends of the derivatives research are discussed in the end.
Key words: andrographolide; derivatives; synthesis; anti-tumor
[1] (a) Gorter, M. K. Recl. Trav. Chim. Pays-Bas Belg. 1912, 30, 151. (b) Cava, M. P.; Chan, W. R.; Haynes, L. J.; Johnson, L. F.; Weinstein, B. Tetrahedron 1962, 18, 397. (c) Fujita, T.; Fujitani, R.; Takeda, Y.; Takaishi, Y.; Yamada, T.; Kido, M.; Miura, I. Chem. Pharm. Bull. 1984, 32, 2117.
[2] Liu, G. L.; Liu, Y. Q. Her. Med. 2006, 25, 48 (in Chinese).(刘国利, 刘永琼, 医药导报, 2006, 25, 48.)
[3] (a) Zhou, J.; Zhang, S. Y.; Ong, C. N.; Shen, H. M. Biochem. Pharmacol. 2006, 72, 132. (b) Bao, G. Q.; Shen, B. Y.; Pan, C. P.; Zhang, Y. J.; Shi, M. M.; Peng, C. H. Toxicol. Lett. 2013, 222, 23.
[4] (a) Cheung, H. Y.; Cheung, S. H.; Li, J.; Cheung, C. S.; Lai, W. P.; Fong, W. F.; Leung, F. M. Planta Med. 2005, 71, 1106.(b) Wong, H. C.; Sagineedu, S. R.; Lajis, N. H.; Loke, S. C.; Stanslas, J. Afr. J. Pharm. Pharmcol. 2011, 5, 225.
[5] (a) Sheeja, K.; Guruvayoorappan, C.; Kuttan, G. Int. Immunopharmacol. 2006, 7, 211.(b) Shen, K.; Ji, L.; Lu, B.; Xu, C.; Gong, C.; Morahan, G.; Wang, Z. Chem.-Biol. Interact. 2014, 218, 99.
[6] Chen, L. L.; Wang, Z. H. Pharm. Today 2010, 20, 41 (in Chinese).(陈伶俐, 王振华, 今日药学, 2010, 20, 41.)
[7] Yu, B. T.; Zhang, Z. R.; Liu, W. S.; Yang, T.; Wang, P. Chin. Tradit. Pat. Med. 2002, 24, 331 (in Chinese). (于波涛, 张志荣, 刘文胜, 杨婷, 王平, 中成药, 2002, 24, 331.)
[8] Thunuguntla, S. S. R.; Nyavanandi, V. K.; Nanduri, S. Tetrahedron Lett. 2004, 45, 9357.
[9] Nanduri, S.; Nyavanandi, V. K.; Thunuguntla, S. S. R.; Kasu, S.; Pallerla, M. K.; Ram, P. S.; Rajagopal, S.; Kumar, R. A.; Ramanujam, R.; Babu, J. M.; Vyas, K.; Devi, A. S.; Reddy, G. O.; Akella, V. Bioorg. Med. Chem. Lett. 2004, 14, 4711.
[10] Nanduri, S.; Pothukuchi, S.; Rajagopal, S.; Akella, V.; Pillai, S. B. K.; Chakrabarti, R. WO 2001085710, 2001 [Chem. Abstr. 2001, 135, 371878].
[11] Nanduri, S.; Rajagopal, S.; Pothukuchi, S.; Pillaj, S. B. K.; Chakrabarti, R. WO 2001057026, 2001 [Chem. Abstr. 2001, 135, 152663].
[12] Jada, S. R.; Subur, G. S.; Matthews, C.; Hamzah, A. S.; Lajis, N. H.; Saad, M. S.; Stevens, M. F. G.; Stanslas, J. Phytochemistry 2007, 68, 904.
[13] Jada, S. R.; Hamzah, A. S.; Lajis, N. H.; Saad, M. S.; Stevens, M. F. G.; Stanslas, J. J. Enzyme Inhib. Med. Chem. 2006, 21, 145.
[14] Hocker, H. J.; Cho, K. J.; Chen, C. Y. K.; Rambahal, N.; Sagineedu, S. R.; Shaari, K.; Stanslas, J.; Hancock, J. K.; Gorfe, A. A. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 10201.
[15] Menon, V.; Bhat, S. Nat. Prod. Commun. 2010, 5, 717.
[16] Sirion, U.; Kasemsook, S.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. Bioorg. Med. Chem. Lett. 2012, 22, 49.
[17] (a) Chen, D. S.; Song, Y. P.; Lu, Y. L.; Xue, X. W. Bioorg. Med. Chem. Lett. 2013, 23, 3166.(b) Xue, X. W.; Li, L.; Song, Y. P.; Lu, Y. L.; Li, J. B. CN 103588738, 2014 [Chem. Abstr. 2014, 160, 356446].
[18] Das, B.; Chowdhury, C.; Kumar, D.; Sen, R.; Roy, R.; Das, P.; Chatterjee, M. Bioorg. Med. Chem. Lett. 2010, 20, 6947.
[19] Preet, R.; Chakraborty, B.; Siddharth, S.; Mohapatra, P.; Das, D.; Satapathy, S. R.; Das, S.; Maiti, N. C.; Maulik, P. R.; Kundu, C. N.; Chowdhury, C. Eur. J. Med. Chem. 2014, 85, 95.
[20] (a) Bao, Q.; Zheng, Y. X.; Chen, L. J. Pract. Oncol. 2008, 23, 590 (in Chinese).(包祺, 郑毅雄, 陈力, 实用肿瘤杂志, 2008, 23, 590.)(b) Xie, Y.; Zhou, X. Y.; Hao, H. P.; Wang, G. P. Acta Physiol. Sin. 2014, 66, 252 (in Chinese).(谢杨, 周雪妍, 郝海平, 王广基, 生理学报, 2014, 66, 252.)
[21] Liu, Z. Y.; Law, W. K.; Wang, D. C.; Nie, X.; Sheng, D. K.; Song, G. R.; Guo, K.; Wei, P.; Ouyang, P. K.; Wong, C. W.; Zhou, G. C. RSC Adv. 2014, 4, 13533.
[22] (a) Hazra, A.; Paira, P.; Sahu, K. B.; Naskar, S.; Saha, P.; Paira, R.; Mondal, S.; Maity, A.; Luger, P.; Weber, M.; Mondal, N. B.; Banerjee, S. Tetrahedron Lett. 2010, 51, 1585.(b) Dey, S. K.; Bose, D.; Hazra, A.; Naskar, S.; Nandy, A.; Munda, R. N.; Das, S.; Chatterjee, N.; Mondal, N. B.; Banerjee, S.; Saha, K. D. PLoS One 2013, 8, e58055.(c) Hazra, A.; Bharitkar, Y. P.; Chakraborty, D.; Mondal, S. K.; Singal, N.; Mondal, S.; Maity, A.; Paira, R.; Banerjee, S.; Mondal, N. B. ACS Comb. Sci. 2013, 15, 41.
[23] Nanduri, S.; Rajagopal, S.; Akella, V. WO 2001085709, 2001 [Chem. Abstr. 2001, 135, 358071].
[24] Fan, Q. Q.; Wang, Q. J.; Zeng, B. B.; Wu, Y. L.; Ji, H. J. China Pharm. Univ. 2010, 41, 326 (in Chinese).(范倩倩, 王秋娟, 曾步兵, 吴玉林, 季晖, 中国药科大学学报, 2010, 41, 326.)
[25] Xu, H.; Huang, W. L.; Zhang, H. B.; Zhou, J. P. Chin. J. Org. Chem. 2005, 25, 1386 (in Chinese).(徐浩, 黄文龙, 张惠斌, 周金培, 有机化学, 2005, 25, 1386.)
[26] (a) Xu, H.; Wang, X. Y.; Huang, W. L.; Zhang, H. B.; Guo, Q. L.; Liu, W. J. China Pharm. Univ. 2005, 36, 496 (in Chinese).(徐浩, 王新扬, 黄文龙, 张惠斌, 郭青龙, 刘娓, 中国药科大学学报, 2005, 36, 496.)(b) Xu, H.; Huang, W. L.; Zhang, H. B.; Guo, Q. L.; Liu, W. Chin. J. Med. Chem. 2005, 15, 212 (in Chinese).(徐浩, 黄文龙, 张惠斌, 郭青龙, 刘娓, 中国药物化学杂志, 2005, 15, 212.)
[27] (a) Zhao, W.; Xu, J. J. Chin. J. Convalescent Med. 2012, 21, 607 (in Chinese).(赵玮, 徐景杰, 中国疗养医学, 2012, 21, 607.) (b) Zheng, L.; Pei, L.; Xia, J. Z. J. Huaihai Med. 2011, 29, 86 (in Chinese).(郑亮, 裴雷, 夏加增, 淮海医药, 2011, 29, 86.)
[28] Li, J.; Huang, W. L.; Zhang, H. B.; Wang, X. Y.; Zhou, H. P. Bioorg. Med. Chem. Lett. 2007, 17, 6891.
[29] (a) Xu, C.; Wang, Z. T. Chin. J. Nat. Med. 2011, 9, 46.(b) Xu, C.; Wang, Z. T. J. China Pharm. Univ. 2011, 42, 29 (in Chinese).(徐冲, 王峥涛, 中国药科大学学报, 2011, 42, 29.)
[30] (a) Liu, H. M.; Xu, H. W.; Wang, H. F. J. Zhengzhou Univ. 2004, 36, 67 (in Chinese).(刘宏民, 徐海伟, 王会芳, 郑州大学学报(理学版), 2004, 36, 67.)(b) Xu, H. W.; Zhang, J. Y.; Liu, H. M.; Wang, J. F. Synth. Commun. 2006, 36, 407.
[31] Dai, G. F.; Xu, H. W.; Liu, H. M.; Dong, R. J.; Yan, L. J.; Zhu, L. P.; Li, W. Y.; Jiang, Z. W.; Wang, Y. N.; Wu, F. J. CN 101972247, 2011 [Chem. Abstr. 2011, 154, 318758].
[32] Xu, H. W.; Jiang, P. J.; Li, W. Y.; Wang, J. F.; Liu, H. M. Chin. J. Chem. 2011, 29, 2114.
[33] Wang, J. G. Exp. Lab. Med. 2011, 29, 459 (in Chinese). (王继贵, 实验与检验医学, 2011, 29, 459.)
[34] Li, J.; Huang, W. L.; Zhang, H. B.; Zhou, H. P. Lett. Drug Des. Discovery 2010, 7, 176.
[35] Wei, S. F.; Tang, Y. B.; Hua, H. M.; Ohkoshi, E.; Goto, M.; Wang, L. T.; Lee, K. H.; Xiao, Z. Y. Bioorg. Med. Chem. Lett. 2013, 23, 4056.
[36] Wang, X. Y.; Xu, H.; Wu, X. M.; Huang, W. L.; Zhou, J. P. J. China Pharm. Univ. 2005, 36, 504.
[37] Li, J.; Huang, W. L.; Zhang, H. B.; Zhou, H. P. J. China Pharm. Univ. 2007, 38, 299.
[38] Liu, H. M.; Xu, H. W.; Liu, G. Z.; Dai, G. F.; Wang, J. F. CN 100999520, 2007 [Chem. Abstr. 2007, 147, 257908].
[39] Nanduri, S.; Nyavanandi, V. K.; Thunuguntla, S. S. R.; Velisoju, M.; Kasu, S.; Rajagopal, S.; Kumar, R. A.; Rajagopalan, R.; Iqbal, J. Tetrahedron Lett. 2004, 45, 4883.
[40] Marcos, I. S.; Moro, R. F.; Carballares, M. S.; Urones, J. G. Tetrahedron Lett. 1999, 40, 2615.
[41] Zhang, D. Y.; Zhou, B.; Wu, J. Q.; Shi, L. Y.; Wang, K.; Wu, X. M.; Hua, W. Y. CN 102653543, 2012 [Chem. Abstr. 2012, 157, 465734].
[42] Liu, G. F.; Fu, Y. Q.; Yang, Z. Q.; Zhao, H. Q.; Fan, X. M. China J. Chin. Mater. Med. 1988, 13, 291 (in Chinese).(刘桂芳, 付玉琴, 杨志强, 赵慧庆, 范雪梅, 中国中药杂志, 1988, 13, 291.)
[43] Zhang, D. Y.; Hai, Y.; Wu, X. M. CN 103804330, 2014 [Chem. Abstr. 2014, 161, 39854].
/
| 〈 |
|
〉 |