

新型羟基功能化离子液体的合成及在Knoevenagel缩合反应中的应用
收稿日期: 2015-01-13
修回日期: 2015-03-19
网络出版日期: 2015-03-24
基金资助
国家科学技术部专项资金(No. 2012BAK30B03); 中央高校基本科研业务费专项资金(Nos. 2232013A3-05, CUSF-DH-D2013048)资助项目.
Synthesis of Novel Hydroxyl-Functionalized Ionic Liquids andApplication in Knoevenagel Condensation
Received date: 2015-01-13
Revised date: 2015-03-19
Online published: 2015-03-24
Supported by
Project supported by the National Science and Technology Ministry (No. 2012BAK30B03) and the Fundamental Research Funds for the Central Universities (Nos. 2232013A3-05, CUSF-DH-D2013048).
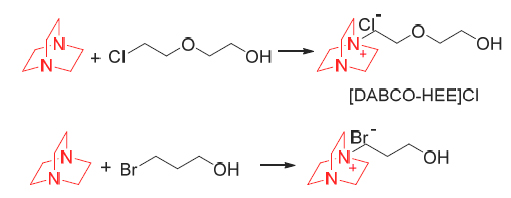
基于1,4-二叠氮双环[2.2.2]辛烷(DABCO)的新型羟基功能化离子液体作为催化剂在无溶剂条件下催化苯甲醛与氰基乙酸乙酯或2,4-噻唑烷二酮的Knoevenagel缩合反应, 在温和反应条件下, 可以较高产率分别得到β-苯基-α氰基丙烯酸乙酯(≥99%)和5-苯乙烯基-2,4-噻唑烷二酮(92%). 这些催化反应操作简单, 产物可从反应混合物中直接分离得到, 并且催化剂表现出较好的重复利用率. 文章最后提出一个可能的反应机理, 并进行了相关讨论.
关键词: 离子液体; 羟基功能化; Knoevenagel缩合反应
刘玉婷 , 李戎 , 邢彦军 . 新型羟基功能化离子液体的合成及在Knoevenagel缩合反应中的应用[J]. 有机化学, 2015 , 35(7) : 1520 -1525 . DOI: 10.6023/cjoc201501013
Knoevenagel condensation of benzaldehyde with active methylene compounds, such as ethylcyanoacetate and 2,4-thiazolidinedione proceeded very smoothly in novel hydroxyl-functionalized ionic liquids based on 1,4-diazabicyclo- [2.2.2]octane (DABCO) under solvent-free conditions and these ionic liquids afforded the products in excellent yields [β-benzyl-α-cyanoacrylicacidethylester (≥99%) and 5-benzylidene-2,4-thiazolidinedione (92%)]. These reactions were operated simply, and the desired products were separated directly from the reaction mixture without further purification. In addition, the ionic liquids used were regenerated and recycled several times without significant loss of activity. Finally, a plausible reaction mechanism was proposed, and the relevant evidence was given.

Key words: ionic liquids; hydroxyl-functionalized; Knoevenagel condensation
[1] Zhao, D.-W; Wu, Y.-T.; Chen, T.-T.; Dai, L.-Y.; Wang, Y.-Y. Chin. J. Org. Chem. 2013, 33, 1791 (in Chinese).(赵东旺, 吴悦彤, 陈婷婷, 戴立益, 王媛媛, 有机化学, 2013, 33, 1791.)
[2] Hou, H.-L.; Li, Z.-F.; Ying, A.-G. Chin. J. Org. Chem. 2014, 34, 1277 (in Chinese).(侯海亮, 李志峰, 应安国, 许松林, 有机化学, 2014, 34, 1277.)
[3] Peter, W.; Wilhelm, K. Angew. Chem., Int. Ed. 2000, 39, 3772.
[4] (a) Plechkova, N. V.; Seddon, K. R. Chem. Soc. Rev. 2008, 37, 123; (b) Tokuda, H.; Tsuzuki, S.; Susan, M. A. B. H.; Hayamizu, K.; Watanabe, M. J. Phys. Chem. B. 2006, 110, 19593.
[5] Fei, Z.-F.; Geldbach, T. J.; Zhao, D.-B.; Dyson, P. J. Chem. Eur. J. 2006, 12, 2122.
[6] Branco, L. C.; Rosa, J. N.; Ramos, J. J. M.; Afonso, C. A. M. A. Chem. Eur. J. 2002, 8, 3671.
[7] Dzyuba, S. V.; Bartsch, R. A. Tetrahedron Lett. 2002, 43, 4657.
[8] Yu, N.; Aramini, J. M.; Germann, M. W.; Huang, Z. Tertrahedron Lett. 2000, 41, 6993.
[9] Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967.
[10] Rumer, J. W.; Dai, S. Y.; Levick, M.; Biniek, L.; Procter, D. J.; McCulloch, I. J. Polym. Sci., Polym. Chem. 2013, 51, 1285.
[11] Han, J.-J.; Xu, Y.-F.; Su, Y.-P.; She, X.-G.; Pan, X.- F. Catal. Commun. 2008, 9, 2077.
[12] Shanthan, R. P.; Venkataratnam, R. V. Tetrahedron Lett. 1991, 32, 5821.
[13] Reddy, B. M.; Patil, M. K.; Rao, K. N.; Reddy, G. K. J. Mol. Catal. A: Chem. 2006, 258, 302.
[14] (a) Hu, Y.; Chen J.; Le Z. G.; Zheng, Q.-G. Synth. Commun. 2005, 35, 739.(b) Yue, C. B.; Mao, A. Q.; Wei, Y. Y.; Lü, M.-J. Catal. Commun. 2008, 9, 1571.
[15] Wu, K.; Li, C.-X. Chin. J. Org. Chem. 2011, 31, 119 (in Chinese).(吴坤, 李存雄, 有机化学, 2011, 31, 119.)
[16] Li, J.-Y.; Peng, J.-J.; Qiu, H.-Y.; Jiang, J.-X.; Wu, J.-R.; Ni, Y.; Lai, G.-Q. Chin. J. Org. Chem. 2007, 27, 483 (in Chinese).(厉嘉云, 彭家建, 邱化玉, 蒋剑雄, 邬继荣, 倪勇, 来国桥, 有机化学, 2007, 27, 483.)
[17] Li, J.; Sun, H.; Cai, X.-C.; Dai, L.-Y. Chin. J. Org. Chem. 2007, 27, 1296 (in Chinese).(李娟, 孙辉, 蔡晓晨, 戴立益, 有机化学, 2007, 27, 1296.)
[18] Morison, D.; Forbes, D. C.; Davis, J. H. Jr. Tetrahedron Lett. 2001, 42, 6053.
[19] Wang, Y.; Shang, Z. C.; Fan, T. X.; Chen, X. J. Mol. Catal. A 2006, 253, 212.
[20] Ying, A.-G.; Ni, Y.-X.; Xu, S.-L.; Liu, S.; Yang, J.-G.; Li, R.-R. Ind. Eng. Chem. Res. 2014, 53, 5678.
[21] AIST: Integrated Spectral Database System of Organic Compounds [Data were obtained from the National Institute of Advanced Industrial Science and Technology (Japan)].
[22] Xu, D.-Z.; Liu, Y.-J.; Shi, S.; Wang, Y.-M. Green Chem. 2010, 12, 514.
[23] Ying, A.-G.; Liu, L.; Wu, G.-F; Chen, X.-Z.; Ye, W.-D.; Chen, J.-H.; Zhang, K.-Y. Chem. Res. Chin. Univ. 2009, 25, 876.
[24] Xu, X.-M.; Li, Y.-Q.; Zhou, M.-Y. Chin. J. Org. Chem. 2004, 24, 1253 (in Chinese).(徐欣明, 李毅群, 周美云, 有机化学, 2004, 24, 1253.)
[25] Shelke, K. F.; Sapkal, S. B.; Madje, B. R.; Shingate, B. B.; Shingare, M. S. Bull. Catal. Soc. Ind. 2009, 8, 30.
[26] Jawale, D. V.; Pratap, U. R.; Lingampalle, D. L.; Mane, R. A. Chin. J. Chem. 2011, 29, 942
[27] Manabendra, S.; Sanchita, R.; Subrata Kumar, C.; Sanjay, B. Green Chem. Lett. Rev. 2008, 1, 113.
[28] Zhang, J.; Zhang, Y.-L.; Zhou, Z.-Q. Green Chem. Lett. Rev. 2014, 7, 90.
/
| 〈 |
|
〉 |