

过渡金属催化法合成平面手性二茂铁衍生物的新进展
收稿日期: 2015-02-23
修回日期: 2015-03-24
网络出版日期: 2015-03-27
基金资助
国家自然科学基金(No. 21202095)、河南省高校科技创新人才基金(No. 14HASTIT016)和山西省自然科学基金(No. 2012011009-4)资助项目.
Recent Advances in Transition-Metal-Catalyzed Enantioselective Syntheses of Planar Chiral Ferrocenes
Received date: 2015-02-23
Revised date: 2015-03-24
Online published: 2015-03-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 21202095), the Program for Science & Technology Innovation Talents in Universities of Henan Province (No. 14HASTIT016) and the Natural Science Foundation of Shanxi Province (No. 2012011009-4).
王艳芳 , 张安安 , 刘澜涛 , 康建勋 , 张富强 , 马文瑾 . 过渡金属催化法合成平面手性二茂铁衍生物的新进展[J]. 有机化学, 2015 , 35(7) : 1399 -1406 . DOI: 10.6023/cjoc201502027
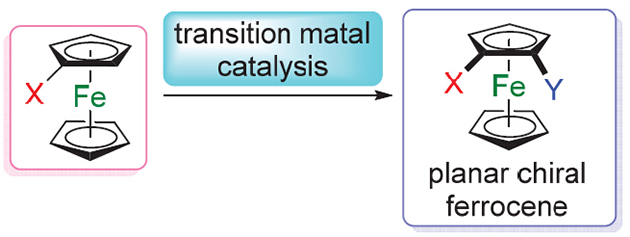
Planar chiral ferrocene derivatives have been proved to be effective ligands and catalysts in asymmetric catalysis. The study toward enantioselective synthesis of planar chiral ferrocenes became one of the high hot spots. In this paper, the recent advance in transition-metal-catalyzed menthods for the syntheses of planar chiral ferrocenes is reviewed.
[1] Carolina, V. B.; Barry, R. S.; Veronika, K.; Maria, M. S.; Constantinos, G. S. J. Organomet. Chem. 2006, 691, 2785.
[2] (a) Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Acc. Chem. Res. 2003, 36, 659. (b) Dai, L.-X.; Hou, X.-L. Chiral Ferrocenes in Asymmetric Catalysis, Wiley-VCH, Weinheim, 2010. (c) Fu, G. C. Acc. Chem. Res. 2004, 37, 542. (d) Fu, G. C. Acc. Chem. Res. 2006, 39, 853.
[3] Schaarschmidt, D.; Lang, H. Organometallics 2013, 32, 5668.
[4] (a) Zhang, J.-L.; Dong, C.-E.; Han, J.; Yu, Z.-L.; Zhang, L.-F. Chin. J. Org. Chem. 2001, 21, 573 (in Chinese).(张俊龙, 董春娥, 韩杰, 于作龙, 张良辅, 有机化学, 2001, 21, 573.) (b) Liu, Z.; He, X. Prog. Chem. 2006, 18, 1489 (in Chinese).(刘振德, 何煦昌, 化学进展, 2006, 18, 1489.) (c) Song, Q.-B.; Dong, Y. Chin. J. Org. Chem. 2007, 27, 66 (in Chinese). (宋庆宝, 东宇, 有机化学, 2007, 27, 66.)(d) Li, G.-H.; Liu, X.-J.; Cheng, H.-B. Chem. Res. 2010, 21, 108 (in Chinese).(黎桂辉, 刘学军, 程红彬, 化学研究, 2010, 21, 108.) (e) Liu, H.-Q.; Shi, L.; Chen, Q.-A.; Wang, L.; Zhou, Y.-G. Acta Chim. Sinica 2013, 71, 40 (in Chinese).(刘洪强, 时磊, 陈庆安, 王磊, 周永贵, 化学学报, 2013, 71, 40.)(f) Su, M.; Li, Q.; Wang, Y.-G.; Chen, S.-F.; Zhao, H.-Y.; Bian, Z.-X. Chin. J. Org. Chem. 2013, 33, 815 (in Chinese).(苏敏, 李晴, 王亚光, 陈树峰, 赵海英, 边占喜, 有机化学, 2013, 33, 815.)(g) Ye, H.-M.; Wang, W.; Zhu, X.-X.; Chen, W.-Q.; Xie, L.-L.; Yuan, Y.-F. Chin. J. Org. Chem. 2013, 33, 827 (in Chinese).(叶宏民, 王薇, 朱晓晓, 陈伟强, 谢莉莉, 袁耀锋, 有机化学, 2013, 33, 827.)
[5] Pla?uk, D.; Zakrzewski, J.; Salmain, M. Org. Biomol. Chem. 2011, 9, 408.
[6] Siegel, S.; Schmalz, H.-G. Angew. Chem., Int. Ed. 1997, 36, 2456.
[7] Duan, W.-L.; Imazaki, Y.; Shintani, R.; Hayashi, T. Tetrahedron 2007, 63, 8529.
[8] Ogasawara, M.; Watanabe, S.; Nakajima, K.; Takahashi, T. J. Am. Chem. Soc. 2010, 132, 2136.
[9] (a)Takebayashi, S.; Shibata, T. Organometallics 2012, 31, 4114. For the related aromatic C-H activation catalyzed by manganese: (b) Zhou, B. W.; Chen, H.; Wang, C.Y. J. Am. Chem. Soc. 2013, 135, 1264.(c) Zhou, B. W.; Ma, P. C.; Chen, H.; Wang, C. Y. Chem. Commun. 2014, 50, 14558.(d) Wang, C. Y. Synlett 2013, 24, 1606.
[10] Shibata, T.; Shizuno, T. Angew. Chem., Int. Ed. 2014, 53, 5410.
[11] (a) Kündig, E. P.; Chaudhuri, P. D.; House, D.; Bernardinelli, G. Angew. Chem., Int. Ed. 2006, 45, 1092. (b) Mercier, A.; Yeo, W. C.; Chou, J.; Chaudhuri, P. D.; Bernardinelli, G.; Kündig, E. P. Chem. Commun. 2009, 5227.(c) Mercier, A.; Urbaneja, X.; Yeo, W. C.; Chaudhuri, P. D.; Cumming, G. R.; House, D.; Bernardinelli, G.; Kündig, E. P. Chem. Eur. J. 2010, 16, 6285.(d) W. C.; Urbaneja, X.; Kundig, E. P. Chimia 2010, 64, 177.
[12] Buchgraber, P.; Mercier, A.; Yeo, W. C.; Besnard, C.; Kündig, E. P. Organometallics 2011, 30, 6303.
[13] Urbaneja, X.; Mercier, A.; Besnard, C.; Kündig, E. P. Chem. Commun. 2011, 47, 3739.
[14] Sokolov, V. I.; Troitskaya, L. L.; Reutov, O. A. J. Organomet. Chem. 1979, 182, 537.
[15] Günay, M. E.; Ilyashenko, G.; Richards, C. J. Tetrahedron: Asymmetry 2010, 21, 2782.
[16] Günay, M. E.; Richards, C. J. Organometallics 2009, 28, 5833.
[17] Xia, J.-B.; You, S.-L. Organometallics 2007, 26, 4869.
[18] Zhang, H.; Cui, X.-L.; Yao, X.-N.; Wang, H.; Zhang, J.-Y.; Wu, Y.-J. Org. Lett. 2012, 14, 3012.
[19] (a) Wencel-Delord, J.; Colobert, F. Chem. Eur. J. 2013, 19, 14010.(b) Zheng, C.; You, S.-L. RSC Adv. 2014, 4 , 6173.
[20] (a) Shi, B. F.; Maugel, N.; Zhang, Y. H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 4882. (b) Shi, B. F.; Zhang, Y. H.; Lam, J. K.; Wang, D. H.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 460.(c) Wasa, M. K.; Engle, M.; Lin, D. W.; Yoo, E. J.; Yu, J. Q. J. Am. Chem. Soc. 2011, 133, 19598. (d) Musaev, D. G.; Kaledin, A.; Shi, B.-F.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 1690.(e) Cheng, X.-F.; Li, Y.; Su, Y.-M.; Yin, F.; Wang, J.-Y.; Sheng, J.; Vora, H. U.; Wang, X.-S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 1236.
[21] Gao, D.-W.; Shi, Y.-C.; Gu, Q.; Zhao, Z.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 86.
[22] Shi, Y.-C.; Yang, R.-F.; Gao, D.-W.; You, S.-L. Beilstein J. Org. Chem. 2013, 9, 1891.
[23] Pi, C.; Li, Y.; Cui, X.-L.; Zhang, H.; Han, Y.-B.; Wu, Y.-J. Chem. Sci. 2013, 4, 2675.
[24] Pi, C.; Cui, X.-L.; Liu, X.-L.; Guo, M.-X.; Zhang, H.-Y.; Wu, Y.-J. Org. Lett. 2014, 16, 5164.
[25] (a) Nishibayashi, Y.; Arikawa, Y.; Ohe, K.; Uemura, S. J. Org. Chem. 1996, 61, 1172.(b) Delacroix, O.; Picart-Goetgheluck, S.; Maciejewski, L.; Brocard, J. Tetrahedron: Asymmetry 1999, 4417.(c) Delacroix, O.; Andriamihaja, B.; Picart-Goetgheluck, S.; Brocard, J. Tetrahedron 2004, 60, 1549. (d) Malfait, S.; Pelinski, L.; Brocard, J. Tetrahedron: Asymmetry 1998, 9, 2207. (e) Picart-Goetgheluck, S.; Delacroix, O.; Maciejewski, L.; Brocard, J. Synthesis 2000, 1421.
[26] (a) Albicker, M. R.; Cramer, N. Angew. Chem., Int. Ed. 2009, 48, 9139.(b) Anas, S.; Cordi, A.; Kagan, H. B Chem. Commun. 2011, 47, 11483. (c) Nakanishi, M.; Katayev, D.; Besnard, C.; Kundig, E. P. Angew. Chem., Int. Ed. 2011, 50, 7438. (d) Martin, N.; Pierre, C.; Davi, M.; Jazzar, R.; Baudoin, O. Chem. Eur. J. 2012, 18, 4480. (e) Saget, T.; Lemouzy, S. J.; Cramer, N. Angew. Chem., Int. Ed. 2012, 51, 2238.
[27] Gao, D.-W.; Yin, Q.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2014, 136, 4841.
[28] Deng, R.-X.; Huang, Y.-Z.; Ma, X.-N.; Li, G.-C.; Zhu, R.; Wang, B.; Kang, Y.-B.; Gu, Z.-H. J. Am. Chem. Soc. 2014, 136, 4472.
[29] Ma, X.-N.; Gu, Z.-H. RSC Adv. 2014, 4, 36241.
[30] Liu, L.-T.; Zhang, A.-A.; Zhao, R.-J.; Li, F.; Meng, T.-J.; Ishida, N.; Murakami, M.; Zhao, W.-X. Org. Lett. 2014, 16, 5336.
/
| 〈 |
|
〉 |