

四棱草中两个新的齐墩果烷型三萜
收稿日期: 2015-03-18
修回日期: 2015-04-13
网络出版日期: 2015-04-17
基金资助
国家自然科学基金(Nos. 30973635, 21302180)资助项目.
Two New Oleanane-Type Triterpenes from Schnabelia oligophylla
Received date: 2015-03-18
Revised date: 2015-04-13
Online published: 2015-04-17
Supported by
Project supported by the National Natural Sciences Foundation of China (Nos. 30973634, 21302180).
杨德兰 , 郭大乐 , 周燕 , 梅文莉 , 肖世基 . 四棱草中两个新的齐墩果烷型三萜[J]. 有机化学, 2015 , 35(8) : 1781 -1784 . DOI: 10.6023/cjoc201503027
Two new oleanane-type triterpenes, named 2β,3β,24-trihydroxyolean-13(18)-ene (1) and 3β-hydroxyolean-13(18)-en-24-oic acid (2) were isolated from the aerial parts of Schnabelia oligophylla. The structures of the new compounds were established by means of HR-ESIMS, 1D and 2D NMR.
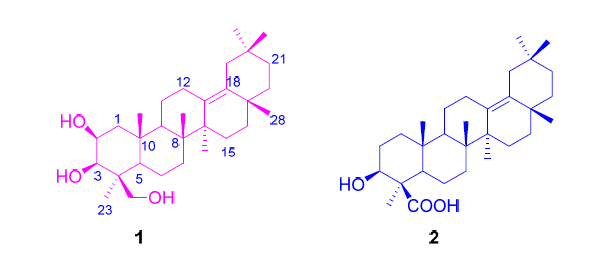
Key words: Schnabelia; Schnabelia oligophylla; oleanane-type; triterpene
[1] Ying, T. S.; Zhang, Y. L.; Boufford, D. E. The Endemic Genera of Seed Plants of China, Science Press, Beijing, 1993 (in Chinese).
(应俊生, 张玉龙, 中国种子植物特有属, 科学出版社, 北京, 1993.)
[2] Wu, Z. Y.; Li, X. W. Flora of China, Vol. 65, Science Press, Beijing, 1977 (in Chinese).
(吴征镒, 李锡文, 中国植物志, 第65卷, 科学出版社, 北京, 1977.)
[3] Dou, H.; Zhou, Y.; Chen, C. X.; Peng, S. L.; Liao, X. J. Nat. Prod. 2002, 65, 1777.
[4] Dou, H.; Liao, X.; Peng, S. L.; Pan, Y. J.; Ding, L. S. Helv. Chim. Acta 2003, 86, 2797.
[5] Dou, H.; Peng, S. L.; Li, B. J.; Luo, S. Z.; Ding, L. S. Chin. J. Org. Chem. 2004, 24, 1469 (in Chinese).
(窦辉, 彭树林, 李帮经, 罗水中, 丁立生, 有机化学, 2004, 24, 1469.)
[6] Dou, H.; Liao, X.; Chen, C. X.; Peng, S. L.; Zhou, Y.; Ding, L. S. Chem. J. Chin. Univ. 2004, 25, 1849 (in Chinese).
(窦辉, 廖循, 陈昌祥, 彭树林, 周燕, 丁立生, 高等学校化学学报, 2004, 25, 1849.)
[7] Dou, H.; Zhou, Y.; Peng, S. L.; Ding, L. S. Chin. Chem. Lett. 2003, 14, 934.
[8] Zhou, Y.; Wang, M. K.; Peng, S. L.; Ding, L. S. Acta Bot. Sinica 2001, 43, 431.
[9] Li, B. J.; Chen, C. X.; Dou, H. Nat. Prod. Res. Dev. 2006, 18, 61 (in Chinese).
(李帮经, 陈昌祥, 窦辉, 李锐, 丁立生, 天然产物研究与开发, 2006, 18, 61.)
[10] Ulubelen, A.; Brieskorn, C. H.; Oezdemir, N. Phytochemistry 1977, 16, 790.
[11] Ahmad, V. U.; Mohammad, F. V.; Rasheed, T. Phytochemistry 1987, 26, 793.
[12] Ageta, H.; Arai, Y.; Suzuki, H.; Kiyotani, T.; Kitabayashi, M. Chem. Pharm. Bull. 1995, 43, 198.
[13] Srivastava, M.; Siddiqui, N. U.; Aslam, M. Orient. J. Chem. 2006, 22, 393.
/
| 〈 |
|
〉 |