

新型三脚架型多苯并咪唑季铵盐类化合物的合成及性能
收稿日期: 2014-12-08
修回日期: 2015-03-25
网络出版日期: 2015-04-30
基金资助
辽宁省教育厅科学技术研究(No. 2009A426)资助项目.
Synthesis and Properties of Novel Tripod Type Multi-benzimidazole Quaternary Ammonium Compounds
Received date: 2014-12-08
Revised date: 2015-03-25
Online published: 2015-04-30
Supported by
Project supported by the Science and Technology Research Program of Liaoning Provincial Department of Education (No. 2009A426).
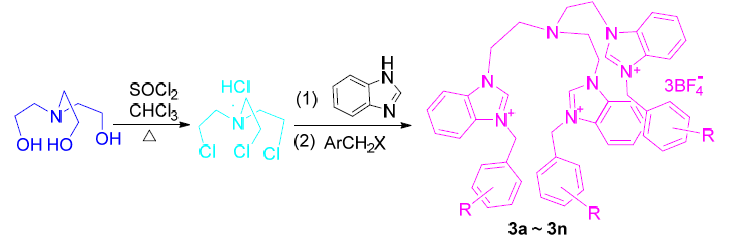
合成了14个具有复合功能的三脚架型多苯并咪唑季铵盐类化合物3a~3n, 并对其进行了结构表征. 探究了目标化合物 3 对 Cdc25B和PTP1B的抑制活性, 结果发现, 目标产物3d、3e、3i、3j和3l对Cdc25B 表现出优良的抑制活性, 其中3d、3e、3j和3l的IC50值低于参照物钒酸钠, 有望成为用于治疗癌症的潜在的Cdc25B 抑制剂; 3d、3e、3i和3l对PTP1B 表现出良好的抑制活性, 3l的IC50值低于参照物齐墩果酸, 有望作为用于治糖尿病潜在的 PTP1B 抑制剂. 研究了生命体系中重要的6种不同阴离子对4个代表性目标化合物的荧光猝灭, 结果发现, I-对目标化合物表现出了优良的猝灭效果, 其中对3c和3e猝灭作用最为明显, 有望成为一种潜在的I-荧光识别探针.
张成路 , 国阳 , 陈莹 , 孙丽杰 , 程安琪 , 赵娜 , 赵宝成 , 唐杰 , 袭焕 . 新型三脚架型多苯并咪唑季铵盐类化合物的合成及性能[J]. 有机化学, 2015 , 35(8) : 1665 -1672 . DOI: 10.6023/cjoc201412014
Fourteen novel tripod type multi-benzimidazole quaternary ammoniums (3a~3n) with complex functions were synthesized for the first time and the structures of the target molecules were well characterized. The inhibitory activities of Cdc25B and PTP1B of 3 were explored. The results showed that 3d, 3e, 3i, 3j and 3l exhibit excellent inhibitory activity against Cdc25B, wherein the IC50 values of 3d, 3e, 3j and 3l were lower than the reference Na3VO4, which were expected to be potential Cdc25B inhibitors for the treatment of cancer. Compounds 3d, 3e, 3i and 3l showed good inhibitory activity against PTP1B, wherein the IC50 value of 3l was lower than the reference oleanolic acid, which may be used as a potential PTP1B inhibitor for the treatment of diabetes. Meanwhile, the fluorescence quenching of six kinds of anions on four target compounds was also studied. It was found that I-exhibited excellent quenching effect, 3c and 3e behaved the best quenching effect and may be a potential recognition fluorescent I-sensor probe.

Key words: benzimidazole; drug activity; fluorescent sensor probe; CDc25B; PTP1B
[1] Song, L.; Xie, Y. Y.; Wang, H. Z. Chin. J. Med. Chem. 2000, 92. (in Chinese). (宋琳, 谢毓元, 王惠贞, 中国药物化学杂志, 2000, 92.)
[2] Meng, J. P.; Lu, Y. H.; Halqam, I.; Zhou, C. H. Chin. J. Biochem. Pharmacol. 2008, 29, 418. (in Chinese). (孟江平, 卢一卉, 海力且木·依不那音, 周成合, 中国生化药物杂志, 2008, 29, 418.)
[3] Guo. Y. C.; Zhou, L. H.; Qiao. Z. P. Chin. J. Inorg. Chem. 2006, 134 (in Chinese). (郭应臣, 卓立宏, 乔占平, 无机化学学报, 2006, 134.)
[4] Ören, I.; Temiz, Ö.; Yalc?n, I.; ?ener, E.; Altanlar, N. Eur. Pharm. Sci. 1989, 7, 153.
[5] Klimešová, V.; Ko??á, J.; Pour, M.; Stachel, J.; Waisser, K.; Kaustová, J. Eur. Med. Chem. 2002, 33, 137.
[6] Ansari, K. F.; Lal, C. Eur. J. Med. Chem. 2009, 44, 2294.
[7] Rajakumara, P.; Sekara, K.; Shanmugaiahb, V.;, Mathivananb, N. Bioorg. Med. Chem. Lett. 2008, 18, 4416.
[8] Beaulieu, P. L.; Bousquet, Y.; Gauthier, J.; Gillard, J.; Marquis, M.; McKercher, G.; Pellerin, C.; Valois, S.; Kukolj, G. J. Med. Chem. 2004, 47, 6884.
[9] Hirashima, S.; Suzuki, T.; Ishida, T.; Noji, S.; Yata, S.; Ando, I.; Komatsu, M.; Ikeda, S.; Hashimoto, H. T. J. Med. Chem. 2006, 49, 4721.
[10] Asthana, S.; Shukla, S.; Vargiu, A. V. Biochemistry 2013, 52, 3752.
[11] Li, Y. F.; Wang, G. F.; He, P. L.; Huang, W. G.; Zhu, F. H. ; Gao, H. Y.; Tang, W.; Luo, Y.; Feng, C. L.; Shi, Li. P.; Ren, Y. D.; Lu, W.; Zuo, J. P. J. Med. Chem. 2006, 49, 4790.
[12] Fang, X. P.; Mei, X. G. Foreign Med. Sci. 2005, 32, 115 (in Chinese). (方学平, 梅兴国, 国外医学, 2005, 32, 15.)
[13] Combs, A. P.; Zhu, W. Y.; Crawley, M. L.; Glass, B.; Polam, P.; Sparks, R. B.; Modi, D.; Takvorian, A.; McLaughlin, E.; Yue, E. W.; Wasserman, Z.; Bower, M.; Wei, M.; Rupar, M.; Ala, P. J.; Reid, B. M.; Ellis, D.; Gonneville, L.; Emm, T.; Taylor, N.; Yeleswaram, S.; Li Y. L.; Richard, W.; Timothy, C. B.; Gregory, H.; Phillip, C. C.; Liu, B. M. J. Med. Chem. 2006, 49, 3774.
[14] Ong, J. X.; Yap, C. W.; Ang, W. H. Inorg. Chem. 2012, 51, 12483.
[15] Erion, M. D.; Dang, Q.; Reddy, M. R.; Kasibhatla, S. R.; Huang, J. W.; Lipscomb, W. N.; Poelje, P. D. J. Am. Chem. Soc. 2008, 129, 15480.
[16] Dang, Q.; Kasibhatla, S. R.; Xiao, W.; Liu, Y.; DaRe, J.; Taplin, F.; Reddy, K. R.; Scarlato, G. R.; Gibson, T.; Poelje, P. D.; Potter, S. C.; Erion, M. D. J. Med. Chem. 2010, 53, 441.
[17] Settimo, F. D.; Primofiore, G.; Settimo, A. D. J. Med. Chem. 2001, 44, 4359.
[18] Ramla, M. M.; Omar, M. A.; El-Khamry, A-M. M.; El-Diwani, H. I. Bioorg. Med. Chem. 2006, 14, 7324.
[19] Rewcastle, G. W.; Gamage, S. A.; Flanagan, J. U. J. Med. Chem. 2011, 54, 105.
[20] Lavecchia, A.; Giovanni, C. D.; Pesapane, A.; Montuori, N.; Ragno, P.; Martucci, N. M.; Masullo, M.; Vendittis, E. D.; Novellino, E. J. Med. Chem. 2012, 55, 4142.
[21] Zhang, C. L.; Wang, X.; Guo, Y.; Wu, Y. F.; Gao, L. N.; Sun, L. J. Chai, J. H.; Zhu, C. A. Chin. J. Org. Chem. 2014, 34, 2331 (in Chinese). (张成路, 王雪, 国阳, 吴一非, 高丽娜, 孙丽杰, 柴金华, 朱长安, 有机化学, 2014, 34, 2331.)
[22] Vladislav, Y. K.; Igor, B. K. ; Vyacheslav, Y. S. Heteroat. Chem. 2005, 16, 492.
[23] Zhang, C. L.; Sun, L. J.; Wu, F. Y.; Qu, R. F.; Zhu, C. A.; Wang, X.; Chai, J. H.; Guo, Y.; Hu, X. Chem. J. Chin. Univ. 2014, 35, 1445 (in Chinese). (张成路, 孙立杰, 武飞宇, 曲瑞峰, 朱长安, 王雪, 柴金华, 国阳, 胡雪, 高等学校化学学报, 2014, 35, 1445.)
[24] Fukuyama, Y.; Tanimura, R.; Maeda, K. Anal. Chem. 2012, 84, 4237.
[25] Singh, N.; Jang, D. O. Org. Lett. 2007, 9, 1991.
[26] Martinez, M.; Scancenon, F. Chem. Rev 2003, 103, 4419.
[27] Kim, Y. H.; Sun, G. Text. Res. J. 2001, 71, 318.
[28] Kar, C.; Basu, A.; Das, G. Tetrahedron 2012, 53, 4754.
[29] Zhang, Y. N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J. Y.; Li, J.; Hua, L. H. Bioorg. Med. Chem. 2008, 16, 8697.
[30] Huang, W. G.; Jiang, Y. Y.; Li, Q.; Li, J.; Li, J. Y.; Lu, W.; Cai, J. C. Tetrahedron 2005, 61, 1863.
[31] Sun, L. P.; Shen, Q.; Piao, H. H.; Ma, W. P.; Gao, L. X.; Zhang, W.; Nan, F. J.; Li, J.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3630.
[32] Alam, M. M.; Nazreen, S.; Haider, S.; Shafi, S.; Yar, M. S.; Hamid, H.; Alam, M. S. Pharm. Chem. Life Sci. 2011, 45, 11
[33] Khatri, V. K.; Chahar, M.; Pavani, K.; Pandey, P. S. J. Org. Chem. 2007, 72, 10224.
[34] Boye, B.; Keramanea, E. M.; Monterob, J. L.; Roquea, J. P. Synth. Commun. 1998, 28, 1737.
[35] Wang, Z. T.; Zhu, L.; Yin, F.; Su, Z. Q.; Li, Z. D.; Li, C. Z. J. Am. Chem. Soc. 2012, 134, 4258.
[36] Matsuo, J.; Iida, D.; Yamanakaa, H.; Mukaiyamaa, T. Tetrahedron 2003, 59, 6739.
[37] Sun, J.; Feng, X. J.; Zhao, Z. R.; Yamamoto, Y.; Bao, M. Tetrahedron 2014, 70, 7166.
[38] Niknam, K.; Kiasat, A. R.; Kazemi, F.; Hossienia, A. Phosphorus, Sulfur, Silicon Relat. Elem.2003, 178, 1385.
[39] Li, Z.; Sheng, C.; Qiu, H.; Zhang, Y. The New J. Org. Synth. 2009, 39, 412.
[40] Zhang, J.; Cui, H. L.; Hojo, M.; Shuang, S. M.; Dong, C. Bioorg. Med. Chem. Lett. 2012, 22, 343.
[41] Caliendoa, G.; Fiorinoa, F.; Perissuttia, E.; Severinoa, B.; Gessib, S.; Cattabrigab, E.; Boreab, P. A.; Santagadaa, V. Eur. J. Med. Chem. 2001, 36, 873.
[42] Boger, D. L.; Palanki, M. S. S. J. Am. Chem. Soc. 1992, 114, 9318.
[43] Tan, H. H.; Ong, W. M.; Lai, S. H. Singapore Med. J. 2007, 48, 582.
/
| 〈 |
|
〉 |