

CytosporoneB的高效合成
收稿日期: 2015-03-30
修回日期: 2015-05-04
网络出版日期: 2015-05-06
基金资助
国家基础研究计划(973 项目, No. 2010CB833802)、国家自然科学基金(Nos. 81373304, 91313303, 81302666)、长江学者和创新团队发展计划(No. IRT13028)和国家杰出青年科学基金(No. 30325044)资助项目.
An Efficient Synthesis of Cytosporone B
Received date: 2015-03-30
Revised date: 2015-05-04
Online published: 2015-05-06
Supported by
Project supported by the National Basic Research Program (973 Program, No. 2010CB833802), the National Natural Science Foundation of China (Nos. 81373304, 91313303, 81302666), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13028) and the National Science Found for Distinguished Young Scholars of China (No. 30325044).
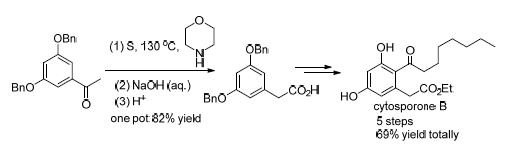
真菌聚酮(cytosporone B, Csn-B)具有多种生物活性, 是研究生物过程的重要小分子探针, 其高效合成备受关注. 本研究以商品3,5-二羟基苯乙酮为原料, 苄基保护羟基得到3,5-二苄氧基苯乙酮, 然后与硫粉和吗啡啉进行Willgerodt-Kindler反应得到3,5-二苄氧基苯乙硫酰胺, 一锅煮水解该酰胺得到关键中间体3,5-二苄氧基苯乙酸; 然后经酯化、傅克酰基化和脱除苄基, 分别以5步反应得到Csn-B(总产率68.8%)或secocurvularin(总收率67.3%); Csn-B经水解得到cytosporone A (Csn-A), 总产率65.3%; Csn-B经还原环合得到cytosporone C (Csn-C), 总产率64.0%. 该合成方法步骤少、产率高, 原料廉价, 适合Csn-B的大量制备.
关键词: 聚酮; cytosporone B; 小分子探针; Willgerodt-Kindler反应
吕超, 李剑芳, 徐洪蛟, 沈月毛 . CytosporoneB的高效合成[J]. 有机化学, 2015 , 35(9) : 2013 -2018 . DOI: 10.6023/cjoc201503049
The fungal polyketide cytosporone B (Csn-B) displays diverse biological activities, and is an important small molecule probe for the studies on biological progress thus that its efficient synthesis attracts much attention. In this study, the commercial available 1-(3,5-dihydroxyphenyl)ethanone was used as starting material. After converting to benzyl ether, the product 1-(3,5-dibenzyloxyphenyl)ethanone was treated with sulfur powder and morpholine followed by hydrolysis in one pot to afford the key intermediate 3,5-dibenzyloxyphenylacetic acid via Willgerodt-Kindler reaction. Respectively, the 3,5-dibenzyloxyphenylacetic acid was converted to Csn-B or secocurvularin (67.3% overall yield) through esterification, Friedel-Crafts acylation reaction and deprotection procedures in 5 steps totally. Cytosporone A (Csn-A) was obtained in 65.3% yield through hydrolysis of Csn-B and cytosporone C (Csn-C) was provided in 64.0% yield via reduction-cyclization of Csn-B. Our synthetic approach to Csn-B and its derivatives has the advantages of short pathways, high yield, economical starting material, and is suitable for large-scale preparation.

[1] Brady, F. S.; Wagenaar, M. M.; Singh, M. P.; Janso, J. E.; Clardy, J. Org. Lett. 2000, 2, 4043.
[2] Zhan, Y. Y.; Du, X. P.; Chen, H. Z.; Liu, J. J.; Zhao, B. X.; Huang, D. H.; Li, G. D.; Xu, Q. Y.; Zhang, M. Q.; Weimer, B. C.; Chen, D.; Cheng, Z.; Zhang, L. R.; Li, Q. X.; Li, S. W.; Zheng, Z. H.; Song, S. Y.; Huang, Y. J.; Ye, Z. Y.; Su, W. J.; Lin, S. C.; Shen, Y. M.; Wu, Q. Nat. Chem. Biol. 2008, 4, 548.
[3] Xia, Z. B.; Cao, X. H.; Rico-Bautista, E.; Yu, J. H.; Chen, L. Q.; Chen, J. B.; Bobkov, A.; Wolf, D. A.; Zhang, X. K.; Dawson, M. I. Med. Chem. Commun. 2013, 4, 332.
[4] Li, J. F.; Lv, C.; Sun, W. Y.; Li, Z. Y.; Han, X. W.; Li, Y. Y.; Shen, Y. M. Antimicrob. Agents Chemother. 2013, 57, 2191.
[5] Palumbo-Zerr, K.; Zerr, P.; Distler, A.; Fliehr, J.; Mancuso, R.; Huang, J.; Mielenz, D.; Tomcik, M.; Fürnrohr, B. G.; Scholtysek, C.; Dees C.; Beyer, C.; Krönke, G.; Metzger, D.; Distler, O.; Schett, G.; Distler, J. H. W. Nat. Med. 2015, 21, 150.
[6] Liu, J. J.; Zeng, H. N.; Zhang, L. R.; Zhan, Y. Y.; Chen, Y.; Wang, Y.; Wang, J.; Xiang, S. H.; Liu, W. J.; Wang, W. J.; Chen, H. Z.; Shen, Y. M.; Su, W. J.; Huang, P. Q.; Zhang, H. K.; Wu, Q. Cancer Res. 2010, 70, 3628.
[7] Wang, W. J.; Wang, Y.; Chen, H. Z.; Xing, Y. Z.; Li, F. W.; Zhang, Q.; Zhou, B.; Zhang, H. K.; Zhang, J.; Bian, X. L.; Li, L.; Liu, Y.; Zhao, B. X.; Chen, Y.; Wu, R.; Li, A. Z.; Yao, L. M.; Chen, P.; Zhang, Y.; Tian, X. Y.; Beermann, F.; Wu, M.; Han, J. H.; Huang, P. Q.; Lin, T. W.; Wu. Q. Nat. Chem. Biol. 2014, 10, 133.
[8] Zhan, Y. Y.; Chen, Y. Y.; Zhang, Q.; Zhuang, J. J.; Tian, M.; Chen, H. Z.; Zhang, L. R.; Zhang, H. K.; He, J. P.; Wang, W. J.; Wu, R.; Wang, Y.; Shi, C. F.; Yang, K.; Li, A. Z.; Xin, Y. Z; Li, T. Y.; Yang, J. Y.; Zheng, Z. H.; Yu, C. D.; Lin, S. C.; Chang, C. S.; Huang, P. Q.; Lin, T. W.; Wu, Q. Nat. Chem. Biol. 2012, 8, 897.
[9] Abrell, L. M.; Borgeson, B.; Crews, P. Tetrhedron Lett. 1996, 37, 8983.
[10] Huang, H.; Zhang, L.; Zhang, X. D.; Ji, X.; Ding, X.; Shen, X.; Jiang, H. L.; Liu, H. Chin. J. Chem. 2010, 28, 1041.
[11] Yoshida, H.; Morishita, T.; Ohshita, J. Chem. Lett. 2010, 39, 508.
[12] Delius, M.; Le, C. M.; Dong, V. M. J. Am. Chem. Soc. 2012, 134, 15022.
[13] Zamberlam, C. E. M.; Meza, A.; Leite, C. B.; Marques, M. R.; Limaa, D. P.; Beatriz, A. J. Braz. Chem. Soc. 2012, 23, 124.
[14] Priebbenow, D. L.; Bolm, Carsten. Chem. Soc. Rev. 2013, 42, 7870.
[15] Vujjini, S. K.; Krishnam, V. R. R. D.; Badarla, K. R.; Vetukuri, V. N. K. V. P. R.; Bandichhor, R.; Kagga, M.; Cherukupally, P. Tetrahedron Lett. 2014, 55, 3885.
[16] Li, X.; Reuman, M.; Russell, R. K.; Adams, R.; Ma, R.; Beish, S.; Branum, S.; Youells, S.; Roberts, J.; Jain, N.; Kanojia, R.; Sui, Z. Org. Process. Res. Dev. 2007, 11, 414.
[17] Bracher, F.; Krauss, J. Nat. Prod. Lett. 1998, 12, 31.
[18] Cai, Z. Y.; Hector, F. D. L. Chin. J. Org. Chem. 2014, 34, 1582 (in Chinese).(蔡祖恽, Hector F. DeLuca, 有机化学, 2014, 34, 1582.)
[19] Alam, M. M.; Adapa, S. R. Synth. Commun. 2003, 33, 59.
[20] Wu, W.; Huang, J. J. Org. Chem. 2014, 79, 10189.
[21] Chen, W.; Hao, H. L.; Zhang, C. L.; Shen, Y. M. Chin. J. Org. Chem. 2014, 34, 797 (in Chinese).(陈旺, 郝慧琳, 张晨露, 沈月毛, 有机化学, 2014, 34, 797.)
/
| 〈 |
|
〉 |