

5-氨基吡唑及5-氨基三唑类成纤维细胞生长因子受体激酶抑制剂的设计合成
收稿日期: 2015-03-27
修回日期: 2015-04-30
网络出版日期: 2015-05-15
基金资助
教育部博士点基金(No. 20130071110071)和上海市科技支撑计划(No. 13431900102)资助项目.
Design and Synthesis of 5-Aminopyrazole and 5-Aminotriazole Derivatives as Fibroblast Growth Factor Receptor Inhibitors
Received date: 2015-03-27
Revised date: 2015-04-30
Online published: 2015-05-15
Supported by
Project supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20130071110071) and the Shanghai Municipal Science & Technology Pillar Program for Bio-pharmaceuticals (No. 13431900102).
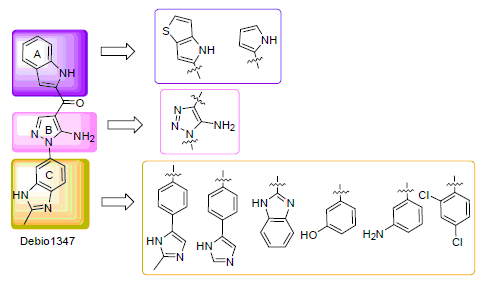
成纤维细胞生长因子受体(FGFR)是近年来抗肿瘤靶向治疗药物研发的前沿热点. 本研究以Debio1347为先导化合物, 依据其与FGFR蛋白的对接结果, 设计并合成了11个5-氨基吡唑及5-氨基-1,2,3-三唑类FGFR抑制剂. 测定了化合物对受体酪氨酸激酶FGFR2以及FGFR2依赖型胃癌细胞株(SNU16)的体外抑制活性, 并对其构效关系进了初步的探讨. 其中, 1-[5-氨基-1-(2-甲基-1H-苯并[d]咪唑-6-基)-1H-吡唑-4-基]-1-(1H-噻吩并[3,2-b]吡咯-2-基)甲酮(8)与1-[5-氨基-1-(2-甲基-1H-苯并[d]咪唑-6-基)-1H-1,2,3-三唑-4-基]-1-(1H-吲哚-2-基)甲酮(18)在酶水平上表现出了与Debio1347 (3.5 nmol·L-1)相近的活性, 其IC50值分别为3.3, 2.3 nmol·L-1; 在细胞水平上, 化合物8和18与Debio1347 (37.7 nmol· L-1)相比较活性略微降低, 其IC50值分别为77.3, 155.2 nmol·L-1.
关键词: 5-氨基吡唑; 5-氨基-1,2,3-三唑; FGFR抑制剂; 抗肿瘤药物
董潜 , 彭霞 , 王雯 , 戴阳 , 赵伟利 , 艾菁 , 董肖椿 . 5-氨基吡唑及5-氨基三唑类成纤维细胞生长因子受体激酶抑制剂的设计合成[J]. 有机化学, 2015 , 35(9) : 1939 -1947 . DOI: 10.6023/cjoc201503045
Fibroblast growth factor receptor (FGFR) is a promising therapeutic target for cancer treatment. A series of 5-aminopyrazole and 5-amino-1,2,3-triazole derivatives were designed as FGFR inhibitors based on the docking mode of Debio1347 within the FGFR kinase domain. The inhibitory effects on FGFR2 kinase and the antitumor activities against human gastric cancer SNU16 cell lines were evaluated. Furthermore, the preliminary structure-activity relationships (SAR) was discussed. The results demonstrated that several compounds present good potency against FGFR2 kinase. 1-(5-Amino-1-(2-methyl-1H-benzo[d]imidazol-6-yl)-1H-pyrazol-4-yl)-1-(1H-thieno[3,2-b]pyrrol-2-yl)methanone (8) and 1-(5-amino-1-(2-methyl-1H-benzo[d]imidazol-6-yl)-1H-1,2,3-triazol-4-yl)-1-(1H-indol-2-yl)methanone (18) were the most potent with IC50 values of 3.3 and 2.3 nmol·L-1, respectively, which are similar to that of Debio1347. Compounds 8 and 18 exhibited slightly weaker antitumor activity against SNU16 cell lines than Debio1347 with IC50 value of 77.3, 155.2 nmol·L-1, respectively.

Key words: 5-aminopyrazole; 5-amino-1,2,3-triazole; FGFR inhibitors; anti-tumor agent
[1] Eswarakumar, V. P.; Lax, I.; Schlessinger, J. Cytokine Growth F. R. 2005, 16, 139.
[2] Turner, N.; Grose, R. Nat. Rev. Cancer 2010, 10, 116.
[3] Brooks, A. N.; Kilgour, E.; Smith, P. D. Clin. Cancer Res. 2012, 18, 1855.
[4] Greulich, H.; Pollock, P. M. Trends Mol. Med. 2011, 17, 283.
[5] Wesche, J.; Haglund, K.; Haugsten, E. M. Biochem. J. 2011, 437, 199.
[6] Courjal, F.; Cuny, M.; SimonyLafontaine, J.; Louason, G.; Speiser, P.; Zeillinger, R.; Rodriguez, C.; Theillet, C. Cancer Res. 1997, 57, 4360.
[7] Kunii, K.; Davis, L.; Gorenstein, J.; Hatch, H.; Yashiro, M.; Di Bacco, A.; Elbi, C.; Lutterbach, B. Cancer Res. 2008, 68, 2340.
[8] Jung, E.-J.; Jung, E.-J.; Min, S. Y.; Kim, M. A.; Kim, W. H. Hum. Pathol. 2012, 43, 1559.
[9] Cappellen, D.; De Oliveira, C.; Ricol, D.; de Medina, S. G. D.; Bourdin, J.; Sastre-Garau, X.; Chopin, D.; Thiery, J. P.; Radvanyi, F. Nat. Genet. 1999, 23, 18.
[10] Dutt, A.; Salvesen, H. B.; Chent, T.-H.; Ramos, A. H.; Onofrio, R. C.; Hatton, C.; Nicoletti, R.; Winckler, W.; Grewal, R.; Hanna, M.; Wyhs, N.; Ziaugra, L.; Richter, D. J.; Trovik, J.; Engelsen, I. B.; Stefansson, I. M.; Fennell, T.; Cibulskis, K.; Zody, M. C.; Akslen, L. A.; Gabriel, S.; Wong, K.-K.; Sellers, W. R.; Meyerson, M.; Greulich, H. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 8713.
[11] Chesi, M.; Nardini, E.; Brents, L. A.; Schrock, E.; Ried, T.; Kuehl, W. M.; Bergsagel, P. L. Nat. Genet. 1997, 16, 260.
[12] Liang, G.; Chen, G.-Z.; Wei, X.-Y.; Zhao, Y.-J.; Li, X.-K. Cytokine Growth Factor Rev. 2013, 24, 467.
[13] Ho, H. K.; Yeo, A. H. L.; Kang, T. S.; Chua, B. T. Drug Discovery Today 2014, 19, 51.
[14] Gavine, P. R.; Mooney, L.; Kilgour, E.; Thomas, A. P.; Al-Kadhimi, K.; Beck, S.; Rooney, C.; Coleman, T.; Baker, D.; Mellor, M. J.; Brooks, A. N.; Klinowska, T. Cancer Res. 2012, 72, 2045.
[15] Guagnano, V.; Furet, P.; Spanka, C.; Bordas, V.; Le Douget, M.; Stamm, C.; Brueggen, J.; Jensen, M. R.; Schnell, C.; Schmid, H.; Wartmann, M.; Berghausen, J.; Drueckes, P.; Zimmerlin, A.; Bussiere, D.; Murray, J.; Porta, D. G. J. Med. Chem. 2011, 54, 7066.
[16] Zhao, G.-S.; Li, W.-Y.; Chen, D.-H.; Henry, J. R.; Li, H.-Y.; Chen, Z.-G.; Zia-Ebrahimi, M.; Bloem, L.; Zhai, Y.; Huss, K.; Peng, S.-B.; McCann, D. J. Mol. Cancer Ther. 2011, 10, 2200.
[17] Nakanishi, Y.; Akiyama, N.; Tsukaguchi, T.; Fujii, T.; Sakata, K.; Sase, H.; Isobe, T.; Morikami, K.; Shindoh, H.; Mio, T.; Ebiike, H.; Taka, N.; Aoki, Y.; Ishii, N. Mol. Cancer Ther. 2014, 13, 2547.
[18] Taka, N.; Ohmori, M.; Takami, K.; Matsushita, M.; Hayase, T.; Hyodo, I.; Kochi, M.; Nishii, H.; Ebiike, H.; Nakanishi, Y.; Mio, T.; Wang, L.; Zhao, W.-L. WO 2011016528, 2011 [Chem. Abstr. 2011, 154, 234696].
[19] Kislyi, V. P.; Danilova, E. B.; Semenov, V. V. Russ. Chem. B 2003, 52, 1770.
[20] Wu, Y.-Y.; Cheng, C.; Jiao, L.-J.; Yu, C.-J.; Wang, S.-F.; Wei, Y.; Mu, X.-L.; Hao, E.-H. Org. Lett. 2014, 16, 748.
[21] Slatt, J.; Romero, I.; Bergman, J. Synthesis-Stuttgart. 2004, 16, 2760.
[22] Hassaneen, H. M. E. Synth. Commun. 2007, 37, 3579.
[23] Novelli, A.; De Santis, A. Tetrahedron Lett. 1967, 3, 265.
[24] Walter, R.; Anderskewitz, R.; Kley, J.; Dollinger, H.; Goeggel, R.; Jung, B.; Mack, J.; Nickolaus, P. WO 2006114371, 2006 [Chem. Abstr. 2006, 145, 471367].
[25] Ishida, T.; In, Y.; Inoue, M.; Kurihara, T.; Moriomoto, K.; Morisaka, K.; Shibata, K. Chem. Pharm. Bull. 1990, 38, 1803.
[26] de Candia, M.; Liantonio, F.; Carotti, A.; De Cristofaro, R.; Altomare, C. J. Med. Chem. 2009, 52, 1018.
[27] Morton, A. A.; Slaunwhite, W. R. J. Biol. Chem. 1949, 179, 259.
[28] Keglevic, D. Croat. Chem. Acta 1961, 33, 83.
[29] Mokrushina, G. A.; Kotovskaya, S. K.; Tyurenkova, G. N.; Ilyenko, V. I.; Platonov, V. G.; Kiseleva, I. V. Khim.-Farm. Zh. 1988, 22, 195.
[30] Neugebauer, W.; Tomanek, M. US 3061435, 1960 [Chem. Abstr. 1960, 54, 73191].
/
| 〈 |
|
〉 |