

过渡金属催化非活化烷基卤化物C(sp3)—C(sp3)交叉偶联反应研究进展
收稿日期: 2015-03-04
修回日期: 2015-05-28
网络出版日期: 2015-06-04
基金资助
国家自然科学基金(Nos. 21472033, 21402036, 21272050)、中国博士后科学基金(No. 2014M551793)和中央高校基本科研业务费专项资金(No. 2013HGBH0046)资助项目.
Recent Advances of Transition Metal-Catalyzed C(sp3)—C(sp3) Cross-Coupling Reactions of Non-activated Alkyl Halides
Received date: 2015-03-04
Revised date: 2015-05-28
Online published: 2015-06-04
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21472033, 21402036, 21272050), the China Postdoctoral Science Foundation (No. 2014M551793), and the Fundamental Research Funds for the Central Universities (No. 2013HGBH0046).
张文曼 , 戴建军 , 许华建 . 过渡金属催化非活化烷基卤化物C(sp3)—C(sp3)交叉偶联反应研究进展[J]. 有机化学, 2015 , 35(9) : 1820 -1833 . DOI: 10.6023/cjoc201503007
Recently, great attention has been directed to the transition-metal-catalyzed cross-coupling reactions of non-acti-vated alkyl halides for the construction of C(sp3)—C(sp3) bonds. However, due to the difficulty of oxidative addition of the non-activated alkyl halides with a metal catalyst and the resulting metal-alkyl intermediate prone to β-H elimination, the cross-coupling of non-activated alkyl halides with alkyl nucleophiles remains a challenge. This review summarizes the recent advances of C(sp3)—C(sp3) cross-coupling of non-activated alkyl halides catalyzed by transition-metals.
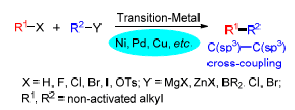
Key words: transition-metal; catalyze; non-activated alkyl halide; cross-coupling; advance
[1] (a) Frisch, A. C.; Beller, M. Angew. Chem., Int. Ed. 2005, 44, 674. (b) Netherton, M. R.; Fu, G. C. Topics in Organometallic Chemistry: Palladium in Organic Synthesis, Ed.: Tsuji, J., Springer, New York, 2005. (c) Netherton, M. R.; Fu, G. C. Adv. Synth. Catal. 2004, 346, 1525. (d) Liu, Y.-L.; Zhang, Y.-H.; Fei, H.-Y.; Jin, X.-H. Chin. J. Org. Chem. 2013, 33, 267 (in Chinese). (刘雅莉, 张韻慧, 费红艳, 晋兴华, 有机化学, 2013, 33, 267.)(e) Liu, Z.-X.; Zhang, Y.; Wang, J.-B. Chin. J. Org. Chem. 2013, 33, 687 (in Chinese). (刘振兴, 张艳, 王剑波, 有机化学, 2013, 33, 687.)(f) Yang, J.; Deng, M.-Z.; Yu, T. Chin. J. Org. Chem. 2013, 33, 693 (in Chinese). (杨军, 邓敏智, 于涛, 有机化学, 2013, 33, 693.)(g) Zhang, Y.; Feng, B.-N. Chin. J. Org. Chem. 2014, 34, 2406 (in Chinese). (张艳, 冯柏年, 有机化学, 2014, 34, 2406.)(h) Wang, B.-Y.; Ni, P.-Z.; Fan, J.-L.; Zheng, H.-D; Zhao, S.-Y.; Bai, Z.-S. Chin. J. Org. Chem. 2014, 34, 2471 (in Chinese). (王碧玉, 倪沛钟, 范吉理, 郑辉东, 赵素英, 白正帅, 有机化学, 2014, 34, 2471.)
[2] (a) Keaton, K. A.; Phillips, A. J. Org. Lett. 2007, 9, 2717. (b) Geist, E.; Kirschning, A.; Schmidt, T. Nat. Prod. Rep. 2014, 31, 441.
[3] Schmidt, T.; Kirschning, A. Angew. Chem., Int. Ed. 2012, 51, 1063.
[4] Li, Z.; Liu, L. Chin. J. Catal. 2015, 36, 3.
[5] Saito, B.; Fu, G. C. J. Am. Chem. Soc. 2007, 129, 9602.
[6] Saito, B.; Fu, G. C. J. Am. Chem. Soc. 2008, 130, 6694.
[7] Owston, N. A.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 11908.
[8] Lu, Z.; Fu, G. C. Angew. Chem., Int. Ed. 2010, 49, 6676.
[9] Lu, Z.; Wilsily, A.; Fu, G. C. J. Am. Chem. Soc. 2011, 133, 8154.
[10] Wilsily, A.; Tramutola, F.; Owston, N. A.; Fu. G. C. J. Am. Chem. Soc. 2012, 134, 5794.
[11] Cordier, C. J.; Lundgren, R. J.; Fu, G. C. J. Am. Chem. Soc. 2013, 135, 10946.
[12] Jiang, X.; Sakthivel, S.; Kulbitski, K.; Nisnevich, G.; Gandelman, M. J. Am. Chem. Soc. 2014, 136, 9548.
[13] (a) Zhou, J.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 14726. (b) Yi, J.; Lu, X.; Sun, Y.-Y.; Xiao, B.; Liu, L. Angew. Chem., Int. Ed. 2013, 52, 12409.
[14] Anderson, T. J.; Jones, G. D.; Vicic, D. A. J. Am. Chem. Soc. 2004, 126, 8100.
[15] Jones, G. D.; McFarland, C.; Anderson, T. J.; Vicic, D. A. Chem. Commun. 2005, 4211.
[16] Csok, Z.; Vechorkin, O.; Harkins, S. B.; Scopelliti, R.; Hu, X. J. Am. Chem. Soc. 2008, 130, 8156.
[17] Vechorkin, O.; Hu, X. Angew. Chem., Int. Ed. 2009, 48, 2937.
[18] Garcia, P. M. P.; Franco, T. D.; Orsino, A.; Ren, P.; Hu, X. Org. Lett. 2012, 14, 4286.
[19] Yu, X.; Yang, T.; Wang, S.; Xu, H.; Gong, H. Org. Lett. 2011, 13, 2138.
[20] Liang, Z.; Xue, W.; Lin, K.; Gong, H. Org. Lett. 2014, 16, 5620.
[21] Lu, X.; Yi, J.; Zhang, Z.-Q.; Dai, J.-J.; Liu, J.-H.; Xiao, B.; Fu, Y.; Liu, L. Chem. Eur. J. 2014, 20, 15339.
[22] Giovannini, R.; Stüdemann, T.; Dussin, G.; Knochel, P. Angew. Chem., Int. Ed. 1998, 37, 2387.
[23] Terao, J.; Watanabe, H.; Ikumi, A.; Kuniyasu, H.; Kambe, N. J. Am. Chem. Soc. 2002, 124, 4222.
[24] Terao, J.; Todo, H.; Watanabe, H.; Ikumi, A.; Kambe, N. Angew. Chem., Int. Ed. 2004, 43, 6180.
[25] Wu, X.; Zhao, Y.; Ge, H. J. Am. Chem. Soc. 2014, 136, 1789.
[26] Ishiyama, T.; Abe, S.; Miyaura, N.; Suzuki, A. Chem. Lett. 1992, 691.
[27] (a) Netherton, M. R.; Dai, C.; Neuschütz, K.; Fu, G. C. J. Am. Chem. Soc. 2001, 123, 10099. (b) Kirchhoff, J. H.; Dai, C.; Fu, G. C. Angew. Chem., Int. Ed. 2002, 41, 1945. (c) Netherton, M. R.; Fu, G. C. Angew. Chem., Int. Ed. 2002, 41, 3910.
[28] Kirchhoff, J. H.; Netherton, M. R.; Hills, I. D.; Fu, G. C. J. Am. Chem. Soc. 2002, 124, 13662.
[29] Zhou, J.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 12527.
[30] Hadei, N.; Kantchev, E. A. B.; O'Brien, C. J.; Organ, M. G. Org. Lett. 2005, 7, 3805.
[31] Hadei, N.; Kantchev, E. A. B.; O'Brien, C. J.; Organ, M. G. J. Org. Chem. 2005, 70, 8503.
[32] O’Brien, C. J.; Kantchev, E. A. B.; Valente, C.; Hadei, N.; Chass, G. A.; Lough, A.; Hopkinson, A. C.; Organ, M. G. Chem.-Eur. J. 2006, 12, 4743.
[33] Peh, G.-R.; Kantchev, E. A. B.; Er, J.-C.; Ying, J. Y. Chem.-Eur. J. 2010, 16, 4010.
[34] Achonduh, G. T.; Hadei, N.; Valente, C.; Avola, S.; O’Brien, C. J.; Organ, M. G. Chem. Commun. 2010, 46, 4109.
[35] McCann, L. C.; Hunter, H. N.; Clyburne, J. A. C.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 7024.
[36] Terao, J.; Naito, Y.; Kuniyasu, H.; Kambe, N. Chem. Lett. 2003, 32, 890.
[37] Chen, X.; Goodhue, C. E.; Yu, J.-Q. J. Am. Chem. Soc. 2006, 128, 12634.
[38] Wang, D.-H.; Wasa, M.; Giri, R.; Yu, J.-Q. J. Am. Chem. Soc. 2008, 130, 7190.
[39] Zhu, R.-Y.; He, J.; Wang, X.-C.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 13194.
[40] Shabashov D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132, 3965.
[41] Zhang, S.-Y.; He, G.; Nack, W. A.; Zhao, Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2013, 135, 2124.
[42] Zhang, S.-Y.; Li, Q.; He, G.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2013, 135, 12135.
[43] Chen, K.; Hu, F.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 3906.
[44] Chen, K.; Shi, B.-F. Angew. Chem., Int. Ed. 2014, 53, 11950.
[45] Cahiez, G.; Chaboche, C.; Jézéquel, M. Tetrahedron 2000, 56, 2733.
[46] Yang, C.-T.; Zhang, Z.-Q.; Liu, Y.-C.; Liu, L. Angew. Chem., Int. Ed. 2011, 50, 3904.
[47] Yang, C.-T.; Zhang, Z.-Q.; Tajuddin, H.; Wu, C.-C.; Liang, J.; Liu, J.-H.; Fu, Y.; Czyzewska, M.; Steel, P. G.; Marder, T. B.; Liu, L. Angew. Chem., Int. Ed. 2012, 51, 528.
[48] Ren, P.; Stern L.-A.; Hu, X. Angew. Chem., Int. Ed. 2012, 51, 9110.
[49] Zhang, Z.-Q.; Yang, C.-T.; Liang, L.-J.; Xiao, B.; Lu, X.; Liu, J.-H.; Sun, Y.-Y.; Marder, T. B.; Fu, Y. Org. Lett. 2014, 16, 6342.
[50] Donkervoort, J. G.; Vicario, J. L.; Jastrzebski, J. T. B. H. R.; Gossage, A.; Cahiez, G.; van Koten, G. J. Organomet. Chem. 1998, 558, 61.
[51] Yang, C.-T.; Zhang, Z.-Q.; Liang, J.; Liu, J.-H.; Lu, X.-Y.; Chen, H.-H.; Liu, L. J. Am. Chem. Soc. 2012, 134, 11124.
[52] Terao, J.; Todo, H.; Begum, S. A.; Kuniyasu, H.; Kambe, N. Angew. Chem., Int. Ed. 2007, 46, 2086.
[53] Shen, R.; Iwasaki, T.; Terao, J.; Kambe, N. Chem. Commun. 2012, 48, 9313.
[54] Kim, J. H.; Chung, Y. K. Chem. Commun. 2013, 49, 11101.
[55] Iwasaki, T.; Imanishi, R.; Shimizu, R.; Kuniyasu, H.; Terao, J.; Kambe, N. J. Org. Chem. 2014, 79, 8522.
[56] Liu, J.-H.; Yang, C.-T.; Lu, X.-Y.; Zhang, Z.-Q.; Xu, L.; Cui, M.; Lu, X.; Xiao, B.; Fu, Y.; Liu, L. Chem.-Eur. J. 2014, 20, 15334.
[57] Someya, H.; Yorimitsu, H.; Oshima. K. Tetrahedron Lett. 2009, 50, 3270.
[58] Zhou, W.; Napoline, J. W.; Thomas, C. M. Eur. J. Inorg. Chem. 2011, 2029.
[59] Hatakeyama, T.; Hashimoto, T.; Kathriarachchi, K. K. A. D. S.; Zenmyo, T.; Seike, H.; Nakamura M. Angew. Chem., Int. Ed. 2012, 51, 8834.
[60] Iwasaki, T.; Takagawa, H.; Singh, S. P.; Kuniyasu, H.; Kambe, N. J. Am. Chem. Soc. 2013, 135, 9604.
[61] Guisan-Ceinos, M.; Tato, F.; Bunuel, E.; Calle, P.; Cardenas, D. J. Chem. Sci. 2013, 4, 1098.
/
| 〈 |
|
〉 |