

南极柳珊瑚代谢物3-Furanoeudesmene的立体选择性全合成及绝对构型确定
收稿日期: 2015-05-01
修回日期: 2015-06-01
网络出版日期: 2015-06-04
基金资助
国家自然科学基金(No. 21172154)资助项目.
Asymmetric Total Synthesis of 3-Furanoeudesmene, A Metabolitefrom Antarctic Gorgonian and Determination of Its AbsoluteConfiguration
Received date: 2015-05-01
Revised date: 2015-06-01
Online published: 2015-06-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172154).
于天姿 , 刘波 . 南极柳珊瑚代谢物3-Furanoeudesmene的立体选择性全合成及绝对构型确定[J]. 有机化学, 2015 , 35(10) : 2157 -2161 . DOI: 10.6023/cjoc201505001
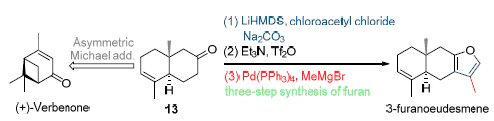
The first total synthesis of 3-furanoeudesmene, an eudesmane-type sesquiterpenoid, is reported herein. Starting from the commercially available (+)-verbenone, the target molecule was achieved stereoselectively over eight steps, with ring closing metathesis and three-step synthesis of furan as the keypoints. The absolute configuration of 3-furanoeudesmene was determined by comparison of the optical rotation value of the synthetic sample with that of the natural product.
Key words: eudesmane; sesquiterpenoid; furan; total synthesis; absolute configuration
[1] Gavagnin, M.; Mollo, E.; Castelluccio, F.; Crispino, A.; Cimino, G. J. Nat. Prod. 2003, 66, 1517.
[2] Nigam, M. C.; Handa, K. L.; Nigam, I. C.; Levi, L. Can. J. Chem. 1965, 43, 3372.
[3] Bowden, B. F.; Braekman, J. C.; Coll, J. C.; Mitchell, S. J. Aust. J. Chem. 1980, 33, 927.
[4] (a) Takagi, S.; Hongo, G. Yakugaku Zasshi 1924, 509, 539.(b) Hikino, H.; Hikino, Y.; Yoshioka, I. Chem. Pharm. Bull. 1962, 10, 641.
[5] Cardona, L.; Garcia, B.; Pedro, J. R.; Rulz, D. Tetrahedron 1994, 50, 5527.
[6] Amorim, A. C. L.; Lima, C. K. F.; Hovell, A. M. C.; Miranda, A. L. P.; Rezende, C. M. Phytomedicine 2009, 16, 923.
[7] (a) Huang, X.; Song, L.; Xu, J.; Zhu, G.; Liu, B. Angew. Chem., Int. Ed. 2013, 52, 952.(b) Yuan, C.; Du, B.; Yang, L.; Liu, B. J. Am. Chem. Soc. 2013, 135, 9291. (c) Du, B.; Yuan, C.; Yu, T.; Yang, L.; Yang, Y.; Liu, B.; Qin, S. Chem. Eur. J. 2014, 20, 2613. (d) Yue, G.; Yang, L.; Yuan, C.; Du, B.; Liu, B. Chin. J. Org. Chem. 2013, 33, 90 (in Chinese).(乐贵洲, 杨立, 袁长春, 杜彪, 有机化学, 2013, 33, 90.)
[8] (a) Honan, M. C. Tetrahedron Lett. 1985, 26, 6393.(b) Bagal, S. K.; Adlington, R. M.; Baldwin, J. E.; Marquez, R. J. Org. Chem. 2004, 69, 9100.
[9] Watanabe, M.; Awen, B. Z.; Kato, M. J. Org. Chem. 1993, 58, 3923.
[10] Kumar, V.; Daum, S. J.; Bell, M. R.; Alexander, M. A.; Christiansen, R. G.; Ackerman, J. H.; Krolski, M. E.; Pilling, C. M.; Jr. Herrmann, J. L.; Winneker, R. C.; Wagner, M. M. Tetrahedron 1991, 47, 5099.
[11] Morice, C.; Garrido, F.; Mann, A.; Suffert, J. Synlett 2002, 501.
[12] (a) Ruprah, P. K.; Cros, J. P.; Pease, J. E.; Whittingham, W. G.; Williams, J. M. M. Eur. J. Org. Chem. 2002, 3145. (b) Paterson, I.; Ng, K. K. H.; Williams, S.; Millican, D. C.; Dalby, S. M. Angew. Chem., Int. Ed. 2014, 53, 2692.(c) Jose, M. S.; Gabriel, M.; Angel, G. R. J. Org. Chem. 1992, 57, 678.
/
| 〈 |
|
〉 |